Novel Schiff base compound taking triphenylamine as center and preparation of novel Schiff base compound
A technology of triphenylamine and Schiff base, which is applied in the field of new Schiff base compounds, can solve the problems of complex sample pretreatment, easy sample loss, high sensitivity reduction, etc., and achieve good promotion prospects, low cost and good repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
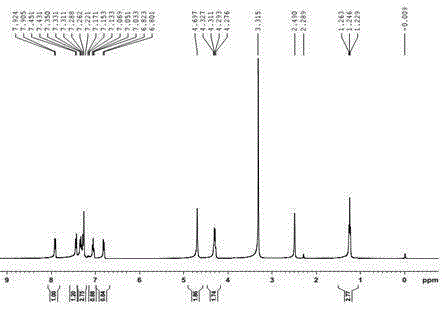
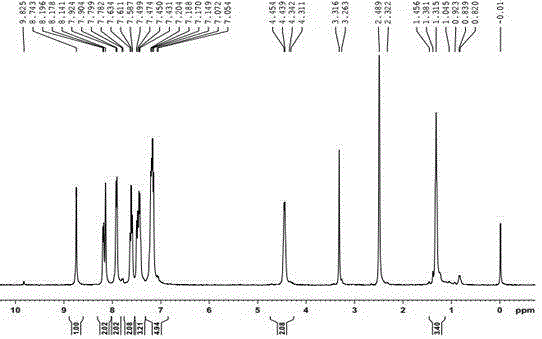
Image
Examples
Embodiment 1
[0051] A novel Schiff base compound centered on triphenylamine, the molecular structural formula is as the aforementioned formula I;
[0052] The preparation method steps are as follows:
[0053] a, the preparation of 4,4-diformyl triphenylamine
[0054] N,N-dimethylformamide (DMF) was dehydrated, and 23ml of N,N-dimethylformamide was added to a dry 100mL three-necked flask, and then magnetically stirred in a cold bath, and gradually dehydrated under the protection of N2 Add 25mLPOCl dropwise 3 After about 1 hour, remove the cold bath and add 5g of triphenylamine, slowly raise the temperature to about 90°C, and after the reaction is over for 3-5 hours, hydrolyze the reactant; then use 1mol.L -1 The NaOH solution was adjusted to a certain pH, and then extracted with the extract, the organic phase was alternately washed with sodium chloride solution and water, and then dewatered with anhydrous magnesium sulfate overnight, filtered with suction, and dichloromethane was removed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com