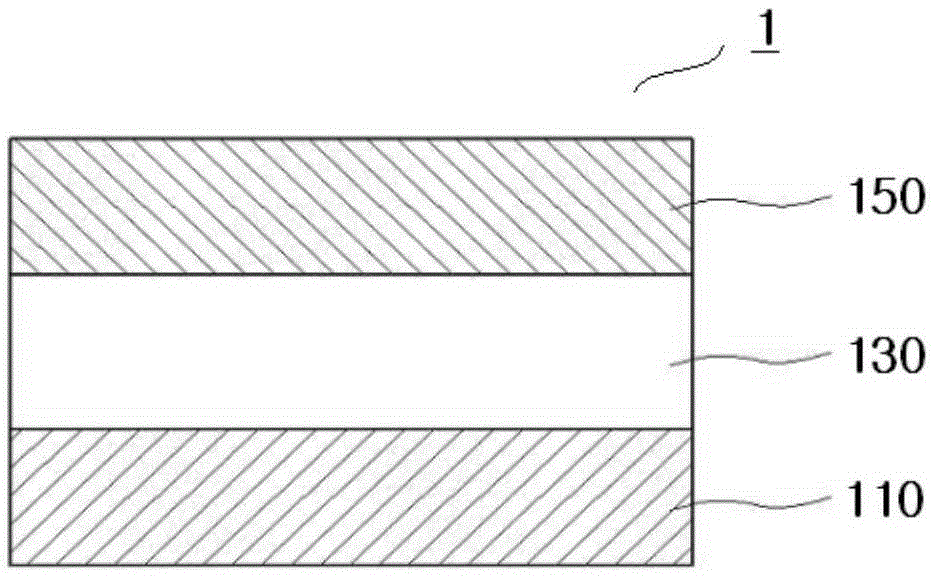
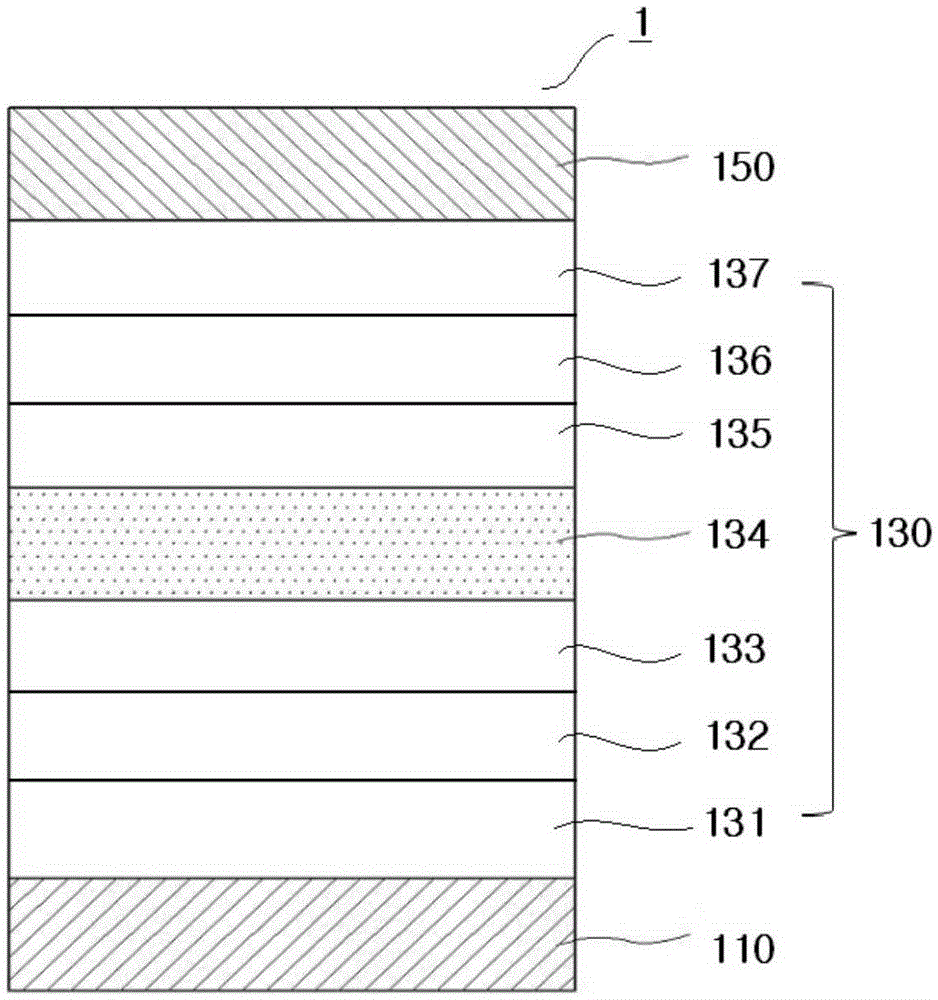
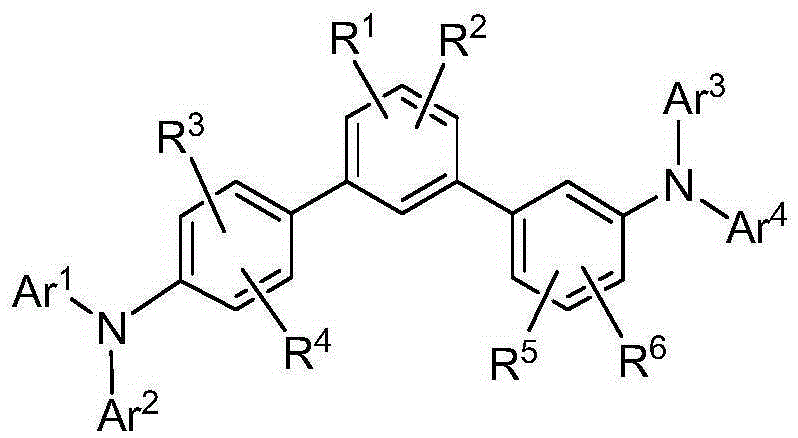
Compound for organic electroluminescent device and organic electroluminescent device including the same
A technology of electroluminescent devices and compounds, applied in the fields of organic semiconductor devices, organic chemistry, electric solid devices, etc., can solve the problems of voltage increase, damage to equipment, deterioration of luminous efficiency of devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0102] Preparation Example 1: Synthesis of Intermediate 1 (3,5-dibromobiphenyl)
[0103]
[0104] Under a nitrogen atmosphere, place 6 g of 1,3-dibromo-5-iodobenzene, 2.2 g of phenylboronic acid, 1.5 g of tetrakis (triphenylphosphine) palladium (0) in a 250 mL three-neck round bottom flask, 4.6g of potassium carbonate, 60ml of toluene and 20ml of methanol, and stirred at 65°C for 4hrs. The reaction solution was cooled, followed by extraction with dichloromethane and water, and the extract solution was concentrated. The solution subjected to column chromatography using a mixed solvent of dichloromethane and n-hexane was concentrated to obtain 6.08 g of 3,5-dibromobiphenyl (yield: 71%).
[0105] 1 HNMR (CDCl 3 ,600MHz)δ7.64(s,2H),7.62(s,1H),7.52-7.50(d,2H),7.46-7.43(dd,2H),7.40-7.38(dd,1H)
preparation Embodiment 2
[0106] Preparation Example 2: Synthesis of Intermediate 2 (3-(2,6-dibromophenyl)-9-phenyl-9H-carbazole)
[0107]
[0108] Under a nitrogen atmosphere, place 6 g of 1,3-dibromo-2-iodobenzene, 2.2 g of N-phenylcarbazole-3-boronic acid, 1.5 g of tetrakis(triphenyl Phosphine)palladium(0), 4.6g of potassium carbonate, 60ml of toluene and 20ml of methanol, and stirred at 65°C for 4hrs. The reaction solution was cooled, followed by extraction with dichloromethane and water, and the extract solution was concentrated. The solution subjected to column chromatography using a mixed solvent of dichloromethane and n-hexane was concentrated to obtain 3.6 g of 3-(2,6-dibromophenyl)-9-phenyl-9H-carbazole (yield: 56%).
[0109] 1 HNMR (CDCl 3 ,600MHz)δ8.13-8.12(d,1H),7.98(s,1H),7.68-7.66(d,2H),7.63-7.60(m,4H),7.49-7.47(m,2H),7.42- 7.40(m,2H),7.33-7.23(m,2H),7.10-7.071(dd,1H)
preparation Embodiment 3
[0110] Preparation Example 3: Synthesis of Intermediate 3 (3,5-bis(diphenylamine)-1-bromobenzene)
[0111]
[0112] Under a nitrogen atmosphere, place 9 g of 1,3,5-tribromobenzene, 8 g of diphenylamine, 9 g of sodium tert-butoxide, and 0.5 g of tris(dibenzylideneacetone) dipalladium in a three-neck round bottom flask (0), 0.3 g of triphenylphosphine and 200 ml of toluene were dissolved and then stirred while maintaining the temperature at 80°C. After the reaction was completed, the reaction solution was extracted with dichloromethane and water, and concentrated. The solution subjected to column chromatography using a mixed solvent of dichloromethane and n-hexane was concentrated to obtain 5 g of 3,5-bis(diphenylamine)-1-bromobenzene (yield: 35%).
[0113] 1 HNMR (CDCl 3 ,600MHz)δ7.24-7.21(t,8H),7.08-7.06(d,8H),7.02-7.00(t,4H),6.75-6.74(d,2H),6.71-6.70(d,1H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com