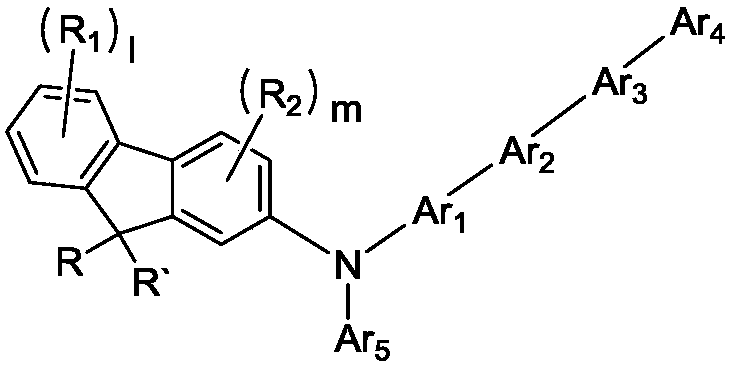
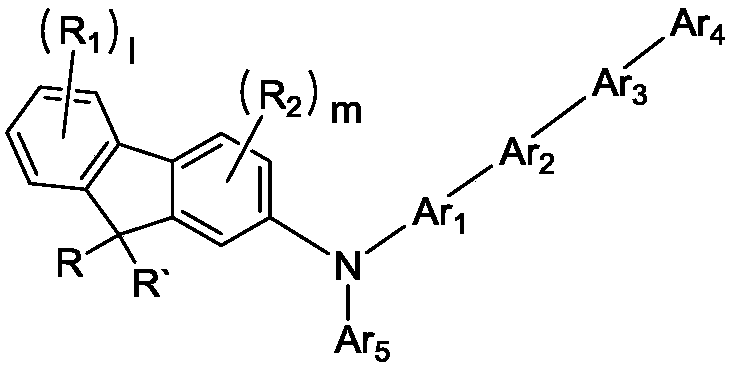
Novel compound and organic electroluminescent device including the same
A technology of organic light-emitting devices and compounds, applied in the field of organic light-emitting devices and novel arylamine compounds, can solve the problems of high driving voltage, short life, low efficiency, etc., achieve long-life devices, prevent recrystallization, and high efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
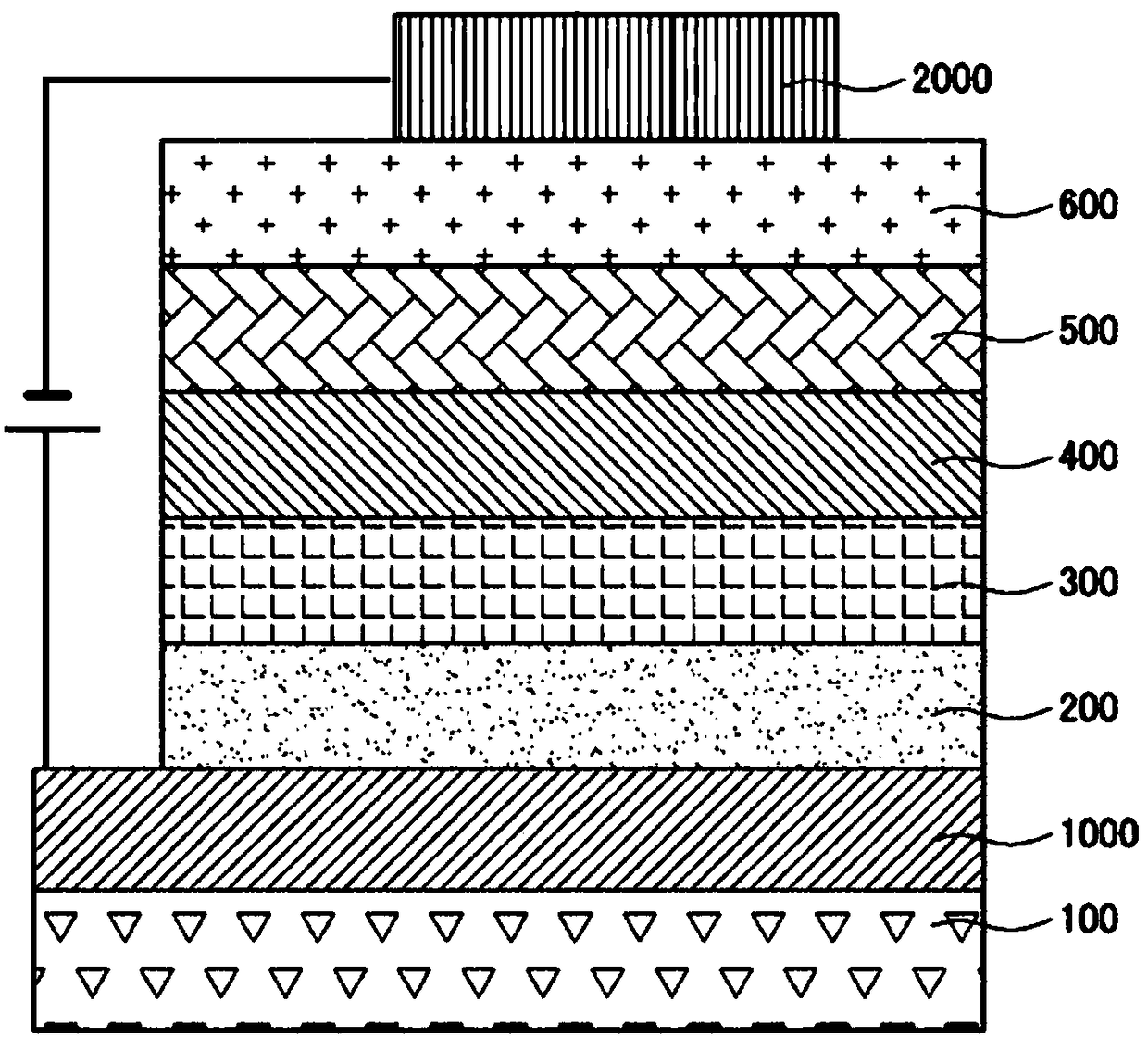
Image
Examples
preparation example 1
[0128] The synthesis of preparation example 1.IM
[0129]
[0130] In order to synthesize the target compound, the preparation of the IM is synthesized by any one of the above-mentioned steps, and may not be limited thereto.
[0131] The synthesis method of the following IM1 is as follows.
[0132]
[0133] In a round bottom flask, dissolve 27.6 g of [1,1'-biphenyl]-4-ylboronic acid, 50.0 g of 4-bromo-4'-iodo-1,1'-biphenyl in 800 ml of 1, After 4-dioxane, put K 2 CO 3 (2M) 210ml and Pd(PPh 3 ) 4 After 4.8 g, reflux and stir. The reaction was confirmed by thin layer chromatography (TLC), and after adding water, the reaction was terminated. The organic layer was extracted with methylcellulose (MC), filtered under reduced pressure, and recrystallized to obtain 50.4 g (yield 74%) of intermediate IM1.
[0134] By the same method as above IM1, using different starting materials, the following IM2 to IM13 were synthesized.
[0135]
[0136]
[0137]
preparation example 2
[0138] Synthesis of Preparation Example 2.OP
[0139] The following synthesis method of OP1 is as follows.
[0140]
[0141] In a round bottom flask, 15.0 g of the above 2-bromo-9,9-dimethyl-9H-fluorene, 5.6 g of aniline, 7.9 g of t-BuONa, Pd 2 (dba) 3 2.0g, (t-Bu) 3 After dissolving 2.5ml of P in 200ml of toluene, reflux and stir. The reaction was confirmed by thin layer chromatography (TLC), and after adding water, the reaction was terminated. The organic layer was extracted with methylcellulose (MC), filtered under reduced pressure, and then recrystallized to obtain 11.75 g (yield 75%) of OP1.
[0142] By the same method as above OP1, using different starting materials, the following OP2 to OP3 were synthesized.
[0143]
[0144]
Synthetic example 1
[0145] Synthesis Example 1. Synthesis of Compound 1
[0146]
[0147] In a round bottom flask, 5.0 g of IM 1, 3.7 g of OP1, 1.9 g of t-BuONa, Pd 2 (dba) 3 0.5g, (t-Bu) 3 After dissolving 0.5 ml of P in 130 ml of toluene, it was stirred under reflux. The reaction was confirmed by thin layer chromatography (TLC), and after adding water, the reaction was terminated. The organic layer was extracted with methylcellulose (MC), and filtered under reduced pressure, followed by column purification and recrystallization to obtain 5.36 g (yield 70%) of Compound 1.
[0148] m / z: 589.28 (100.0%), 590.28 (49.1%), 591.28 (11.8%), 592.29 (1.8%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com