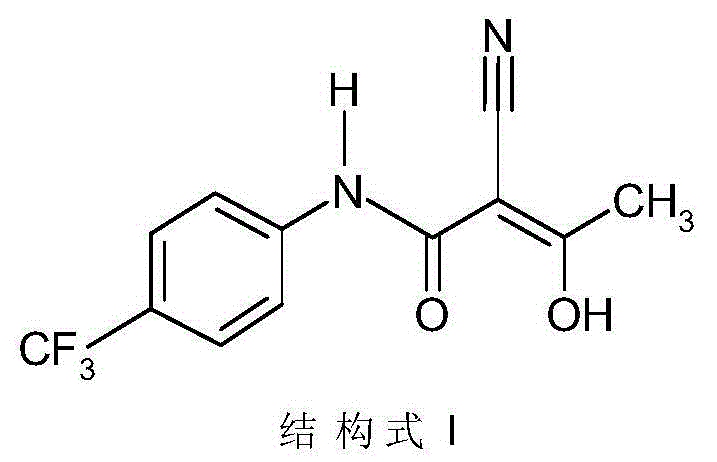
Environmental-protection simple preparation method of teriflunomide
A technology of fluomibide and leflunomide, which is applied in the field of preparation of terimide, can solve the problems of increasing the cost of post-processing of industrial waste, and achieve the effects of convenient preparation method, short reaction steps, and easy industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of Terimide of the present invention uses eflunomide as a raw material to prepare Terimide through a ring-opening reaction and an acidification reaction, wherein the ring-opening reaction is carried out in an aqueous alkali solution.
[0027] The time for the ring-opening reaction is 1.5-4 hr, preferably 2-3 hr.
[0028] The method also includes the step of recrystallizing the tirimide obtained after the acidification reaction to obtain pure tirimide.
[0029] Described alkali is sodium hydroxide, potassium hydroxide.
[0030] The weight ratio of the leflunomide to the alkali aqueous solution is 1:15-1:35.
[0031] The weight ratio of the leflunomide to the alkali aqueous solution is 1:20-1:30.
[0032] The concentration of the alkali aqueous solution is 0.3-0.7 mol / L.
[0033] The concentration of the alkali aqueous solution is 0.4-0.6 mol / L, preferably 0.5 mol / L.
[0034] The temperature of the ring-opening reaction is 15-50°C.
[0035] The...
Embodiment 1
[0063] Preparation of Terimide
[0064] Weigh 9.6 g of sodium hydroxide, dissolve it in 480 ml of water at room temperature, and put 20 g of leflunomide into the sodium hydroxide solution. After stirring at room temperature for 2 hours, the end point of the reaction was determined by thin-layer chromatography. Add 240ml of water and continue stirring for 30min, and filter the insoluble matter.
[0065] Add 23.4ml of concentrated hydrochloric acid to the filtrate for acidification, and a white solid precipitates out immediately. Filter and dry to obtain the crude product of Terimite. The yield is 90-98%.
[0066] Add 240 ml of ethyl acetate to 15 g of the crude product of Terimide, reflux until dissolved, and cool down to crystallize. Filter and dry to obtain the pure product of Terimide with a purity ≥99.8% (HPLC).
[0067] MP: 228-232°C
[0068] Mass: 269(M + -1)
[0069] H-NMR(DMSO): δ2.265(s, 3H); 7.68(d, 2H); 7.76(d, 2H); 10.83(s, 1H)
[0070] IR: 3302, 2220, 1634...
Embodiment 2
[0072] Preparation of Terimide
[0073] Weigh 13.4 g of potassium hydroxide, dissolve it in 480 ml of water at room temperature, and put 20 g of leflunomide into the solution. After reacting at room temperature for 2 hours, TLC determined the reaction end point. Add 240ml of water and continue stirring for 30min, and filter the insoluble matter.
[0074] Add 23.4ml of concentrated hydrochloric acid to the filtrate for acidification, and a white solid precipitates out. Filter and dry to obtain the crude product of Terimite. The yield was 95-98%.
[0075] After recrystallization, the purity of Terimide is ≥99.8% (HPLC).
[0076] MP: 228-232°C
[0077] Mass: 269(M + -1)
[0078] H-NMR(DMSO): δ2.265(s, 3H); 7.68(d, 2H); 7.76(d, 2H); 10.83(s, 1H)
[0079] IR: 3302, 2220, 1634, 1593, 1552, 1419, 1324, 1247, 1158, 1114, 1072, 971, 843, 686cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com