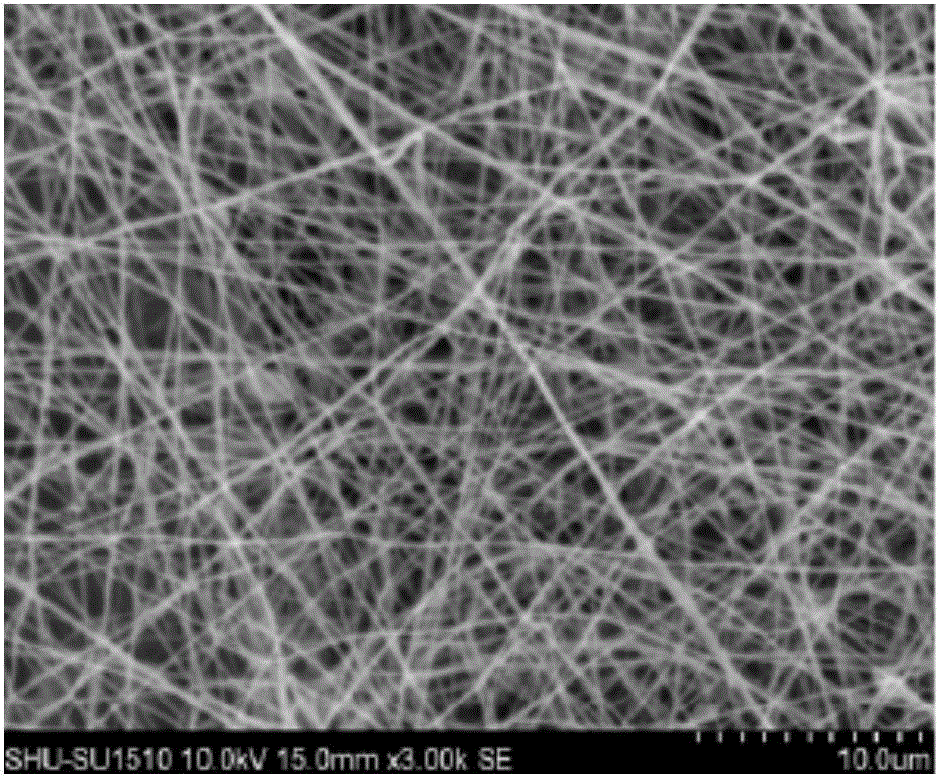
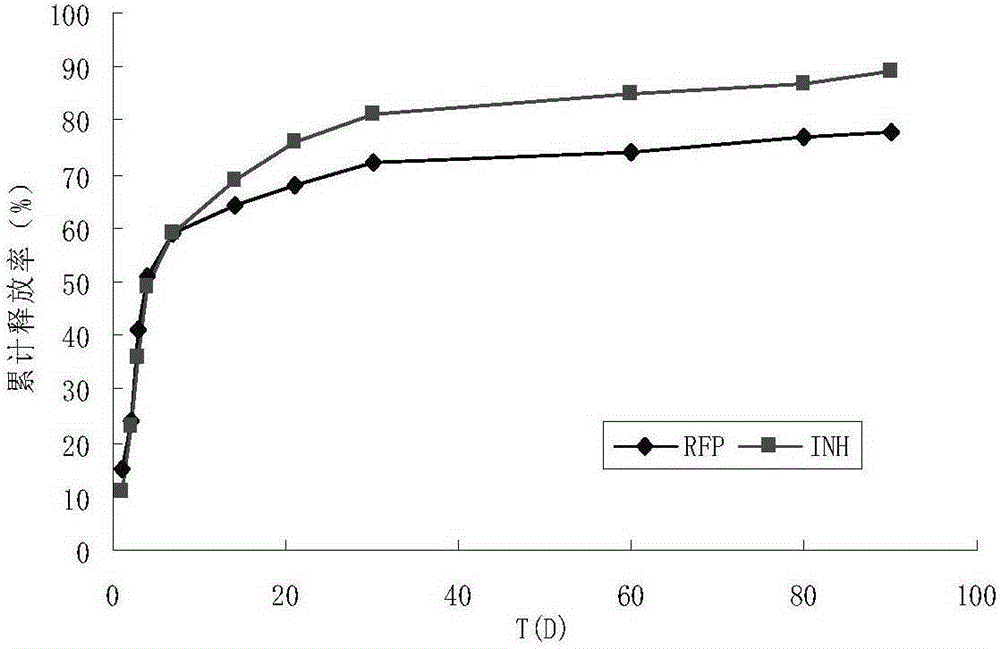
Bone tissue engineering stent and preparation method thereof
A technology of bone tissue engineering and bone powder, applied in additive processing, medical science, prosthesis, etc., can solve problems such as liver, kidney and other organ damage and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Dissolve PLGA10g (25:75, w / w) PLGA in 100ml of dichloromethane and acetone mixed solution, wherein dichloromethane: acetone (v / v) = 1:1, and make a 10% PLGA solution.
[0031] (2) Add 15 g of 5 μm allogeneic bone powder gradually to 10% PLGA solution in small amounts at 20° C., and stir with sealed magnetic force for 3 hours until a uniform mixed solution is formed.
[0032] (3) 3 g of isoniazid (INH), 1 g of rifampicin (RFP), and 1 g of bone morphogenetic protein (BMP-2) were added to the above mixed solution, sealed and ultrasonically stirred for 1 hour to prepare a spray solution.
[0033] (4) In order to obtain a suitable viscosity for printing, transfer the solution into a syringe and pre-cool at 4°C for 30 minutes.
[0034] (5) Place the pneumatic 3D printer in an ultra-clean bench and irradiate it with ultraviolet light for 30 minutes to sterilize it. Install the pre-cooled injection needle tube filled with printing fluid on the printer frame, and run the pr...
Embodiment 2
[0036] (1) PLGA is dissolved in dichloromethane (DCM) and acetone to form a 10%-25% solution. For example, dissolve 30g (85:15, w / w) PLGA in 200ml dichloromethane and acetone mixed solution, wherein dichloromethane: acetone (v / v) = 1:1, and make a 15% PLGA solution.
[0037] (2) 15g of 7 μm allogeneic bone powder was gradually added to 15% PLGA solution in a small amount at 20° C., sealed and magnetically stirred for 3 hours until a uniform mixed solution was formed.
[0038] (3) 4 g of isoniazid (INH), 3 g of rifampicin (RFP), and 2 g of bone morphogenetic protein (BMP-2) were added to the above mixed solution, sealed and ultrasonically stirred for 1 hour to prepare a spray solution.
[0039](4) In order to obtain a suitable viscosity for printing, transfer the solution into a syringe and pre-cool at 4°C for 30 minutes.
[0040] (5) Place the pneumatic 3D printer in an ultra-clean bench and irradiate it with ultraviolet light for 30 minutes to sterilize it. Install the pre-...
Embodiment 3
[0042] (1) PLGA is dissolved in dichloromethane (DCM) and acetone to form a 10%-25% solution. For example, 30g (50:50, w / w) of PLGA was dissolved in 300ml of dichloromethane and acetone mixed solution, wherein dichloromethane: acetone (v / v) = 1:1, made into 10% PLGA solution.
[0043] (2) 30 g of 7 μm allogeneic bone powder was gradually added to the 10% PLGA solution in small amounts at 20° C., sealed and magnetically stirred for 3 hours until a uniform mixed solution was formed.
[0044] (3) 2 g of isoniazid (INH), 4 g of rifampin (RFP), and 2 g of bone morphogenetic protein (BMP-2) were added to the above mixed solution, sealed and ultrasonically stirred for 1 hour to prepare a spray solution.
[0045] (4) In order to obtain a suitable viscosity for printing, transfer the solution into a syringe and pre-cool at 4°C for 30 minutes.
[0046] (5) Place the pneumatic 3D printer in an ultra-clean bench and irradiate it with ultraviolet light for 30 minutes to sterilize it. Ins...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com