Stable compositions comprising heparinoid, acute-acting anesthetic, and buffer
A technology of buffer solution and composition, applied in the field of stable composition, can solve the problems of reducing the curative effect of the composition, reducing the bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Composition comprising heparin, lidocaine and bicarbonate
[0129] Heparin (50,000 units or 250 mg plus lidocaine (200 mg), buffered with sodium bicarbonate to pH 7.5 and a final volume of 15 ml. After 12 months and 18 months, both heparin and lidocaine remain Stability above 95%.
Embodiment 2
[0131] Composition comprising heparin, lidocaine and phosphate
[0132] (Predictive Example)
[0133] Heparin (50,000 units or 250 mg plus lidocaine (200 mg), buffered with phosphate to pH 7.5 and a final volume of 15 ml. After 12 months and 18 months, both heparin and lidocaine remain at Stability is above 95%.
Embodiment 3
[0135] Stability and absorption rate of composition including heparin, lidocaine and phosphate
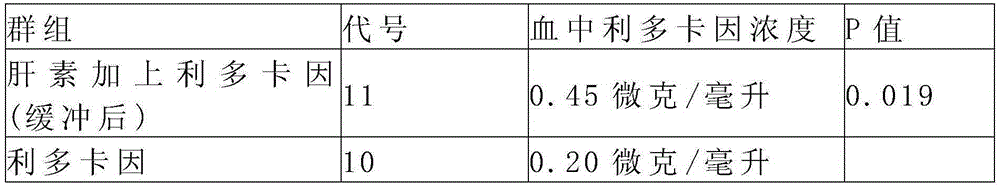
[0136] Clinical trials are used to evaluate the stability and absorption rate of a composition comprising heparin, lidocaine and phosphate relative to lidocaine alone. Based on the standard of lidocaine concentration in the blood, a 25 ml solution composed of heparin and lidocaine includes 333 mg of lidocaine hydrochloride and 50,000 units of heparin, buffered with phosphate to a pH of 7.1-7.2, Available at professional compound pharmacies. For lidocaine, a 25 ml solution prepared with lidocaine hydrochloride contains 333 mg of lidocaine hydrochloride and has a pH of approximately 6.3 (unbuffered). The above-mentioned products were injected into the urethra and bladder of patients with interstitial cystitis, and blood was drawn 45 minutes later to measure the concentration of lidocaine in the blood, and the concentration of lidocaine was measured by high performance liquid chromatogr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com