Method for improving iodometry measurement accuracy of samples containing interfering substances
An interfering substance, iodometric method, applied in the field of analysis and detection, can solve problems such as difficulty in accurate determination, and achieve the effect of easy operation and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1. Direct iodometric method measures vitamin C in a certain sample
[0041] The normal method is:
[0042] Accurately weigh an appropriate amount of vitamin C-containing sample (or prepare an aqueous solution of appropriate concentration in advance), add appropriate amount of pure water, add 10mL 2mol / L acetic acid and 1mL 1% starch solution, and immediately titrate with 0.05mol / L iodine standard solution to a stable Light blue, no fading within 30 seconds is the end point. Record the volume of iodine standard solution consumed. At the same time, titrate the reagent blank with pure water, and record the volume of iodine standard solution consumed by the blank.
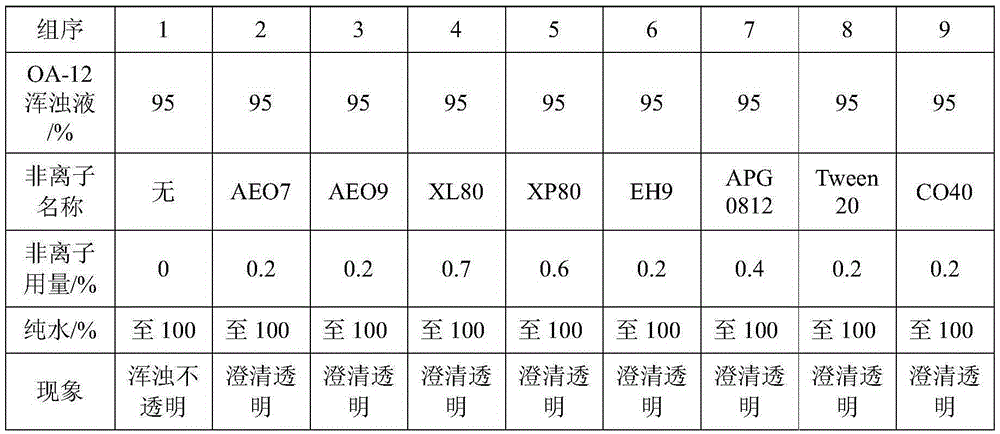
[0043] When the sample contains interfering substances (OA-12), the titration end point is turbid with the above method, and the starch cannot develop color. The following improved methods can be used to solve the problem:
[0044] Accurately weigh an appropriate amount of vitamin C-containing sam...
Embodiment 2
[0045] Embodiment 2. residual iodometric method measures formaldehyde in a certain sample
[0046] The normal method is:
[0047] Accurately weigh an appropriate amount of formaldehyde-containing sample (or prepare an aqueous solution with a suitable concentration in advance), add 20.00mL of 0.1mol / L iodine solution and 15mL of 1mol / L sodium hydroxide solution, and let it stand for 15min. Add 20mL of 0.5mol / L sulfuric acid solution and let it stand for 15min. Titrate with 0.1mol / L sodium thiosulfate standard solution until the solution is light yellow, add 1mL of 0.5% starch solution and continue titrating until the blue just fades as the end point. Record the volume of sodium thiosulfate standard solution consumed. At the same time, titrate the reagent blank with pure water, and record the volume of sodium thiosulfate standard solution consumed by the blank.
[0048] When the sample contains interfering substances (1227), use the above method to titrate the end point to ...
Embodiment 3
[0050] Embodiment 3: the available chlorine of certain disinfectant is measured by displacement iodometric method
[0051] The normal method is:
[0052] Accurately weigh an appropriate amount of chlorine-containing disinfectant (or prepare an aqueous solution with a suitable concentration in advance) in a 100mL iodine bottle, add 10mL of 2mol / L sulfuric acid, 10mL of 100g / L potassium iodide solution, and place in a dark place for 5 minutes. Titrate with 0.1mol / L sodium thiosulfate standard solution until the solution is light yellow, add 10 drops of 5g / L starch solution, continue titrating until the blue color disappears, record the volume of consumed sodium thiosulfate standard solution. At the same time, titrate the reagent blank with pure water, and record the volume of sodium thiosulfate standard solution consumed by the blank.
[0053] When the sample contains interfering substances (OA-12), the titration end point is turbid with the above method, and the starch canno...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com