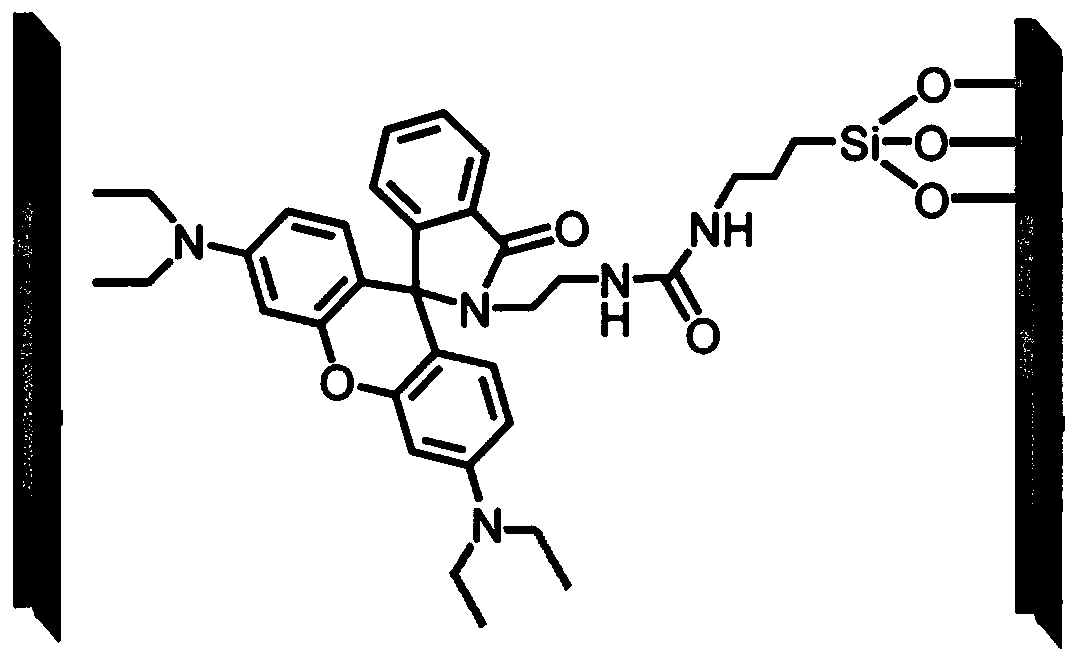
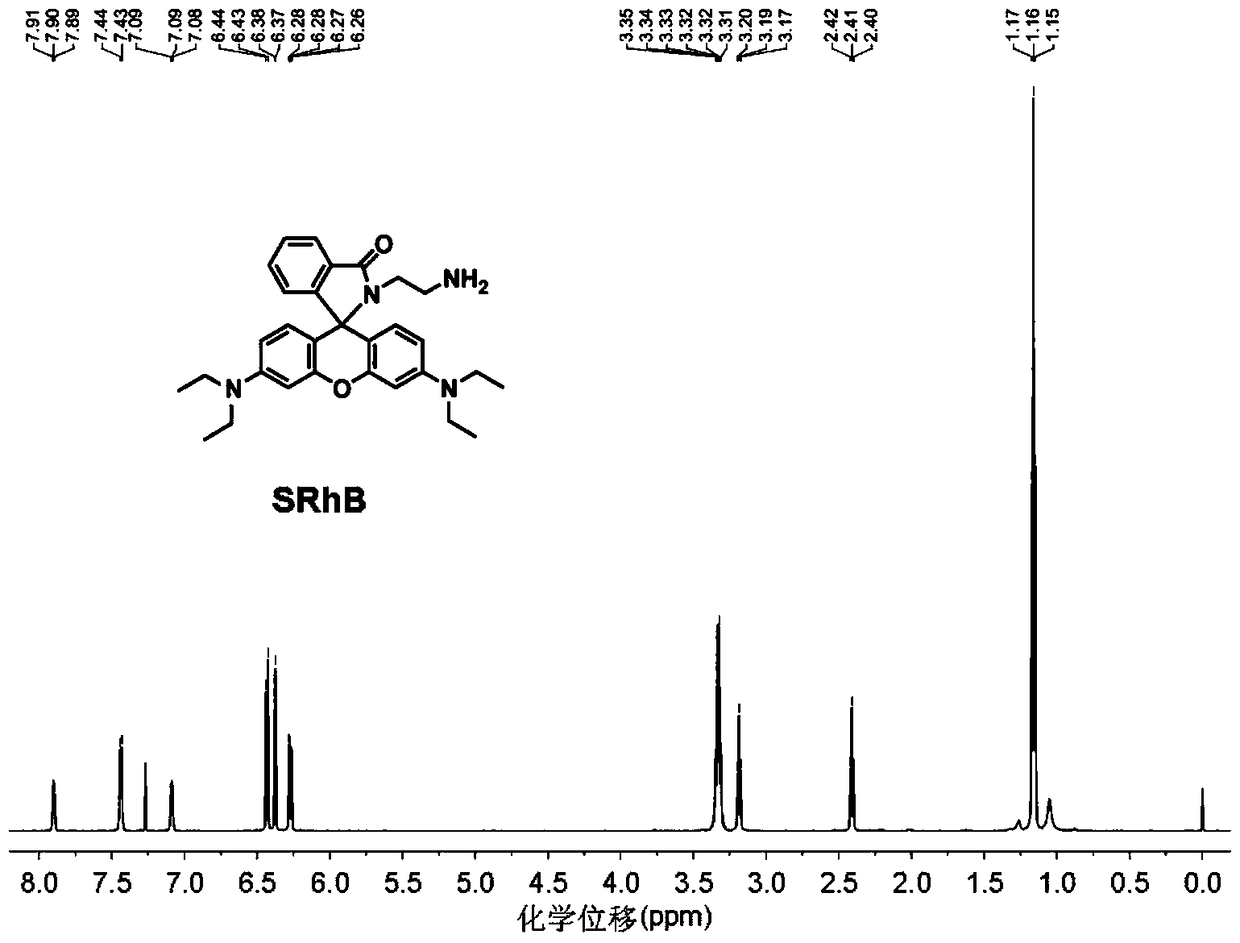
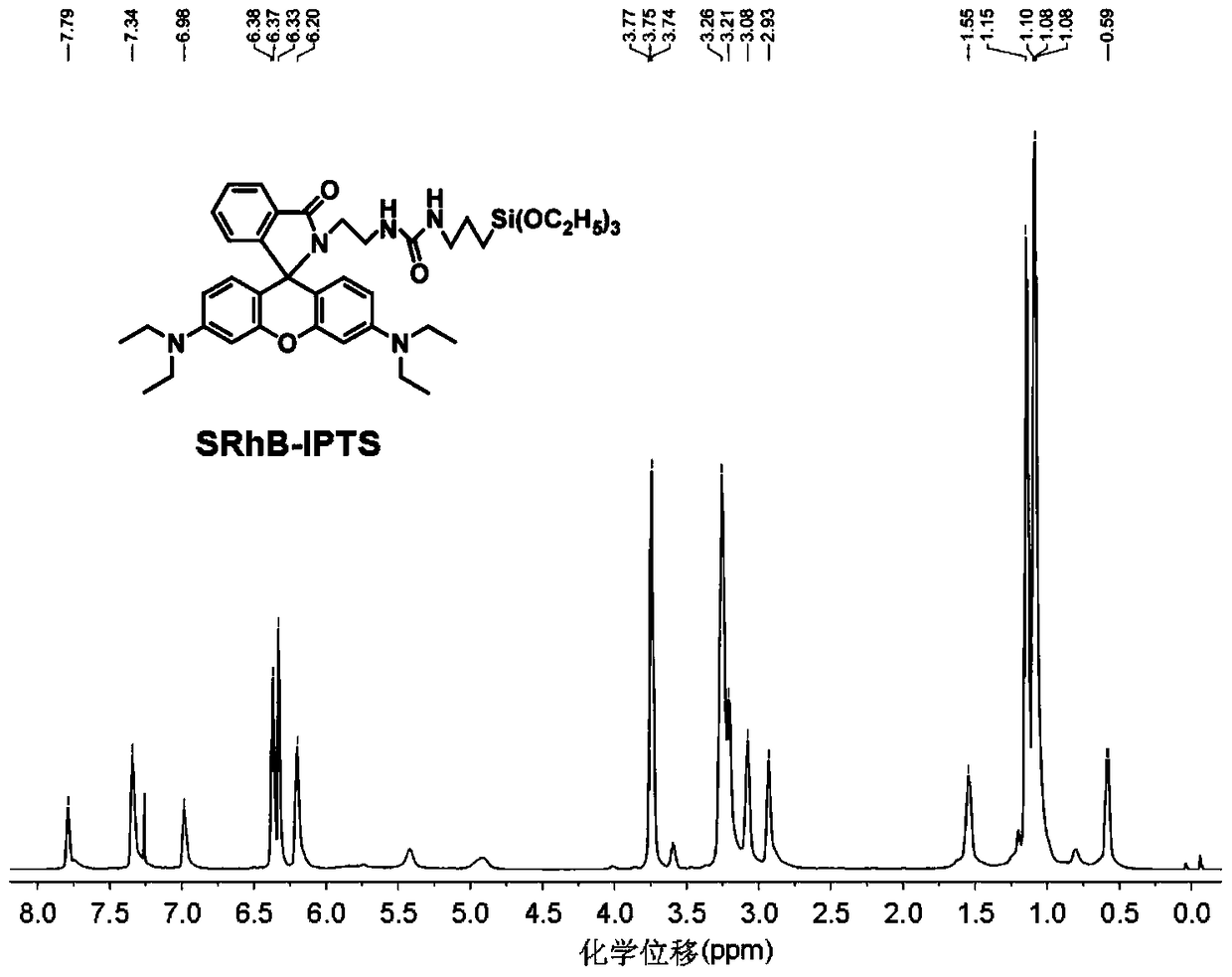
A kind of immobilized rhodamine b-based fluorescence sensor and preparation method thereof
A fluorescence sensor and sensor technology, applied in the field of functional materials/biochemical sensing, can solve the problems of decreased specific surface area and pore volume of mesoporous molecular sieve immobilized fluorescence sensor, damaged carrier surface topography, and low structural stability, etc. Achieve good selective recognition effect, fast detection speed, and stable structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthetic experiments were carried out in an oil bath. In a 250mL three-neck flask, completely dissolve 5.0g (10.46mmol) Rhodamine B in 180mL 40°C absolute ethanol, then quickly add 12.56g (209mmol) ethylenediamine liquid, slowly raise the oil bath to 85°C, The reaction was refluxed for 24 hours until the solution changed from fluorescent red to light yellow. After the reaction was complete, the solvent was removed by rotary evaporation to obtain a solid mixture, which was then extracted with 100 mL of dichloromethane and 100 mL of water, and the organic layer was repeatedly washed with deionized water (100 mL) five times to remove residual rhodamine B. Liquid separation to obtain an organic layer, anhydrous MgSO 4 After drying, filtering, and rotary evaporation to remove dichloromethane, a light orange crude product was obtained. Subsequently, it was recrystallized with acetonitrile and dried in a vacuum oven at 60° C. for 6 hours to obtain 3.23 g of a refined light ...
Embodiment 2
[0056] Synthetic experiments were carried out in an oil bath. In a 250mL three-neck flask, completely dissolve 5.0g (10.46mmol) Rhodamine B in 180mL 40°C absolute ethanol, then quickly add 12.56g (209mmol) ethylenediamine liquid, slowly raise the oil bath to 85°C, The reaction was refluxed for 24 hours until the solution changed from fluorescent red to light yellow. After the reaction was complete, the solvent was removed by rotary evaporation to obtain a solid mixture, which was then extracted with 100 mL of dichloromethane and 100 mL of water, and the organic layer was repeatedly washed with deionized water (100 mL) five times to remove residual rhodamine B. Liquid separation to obtain an organic layer, anhydrous MgSO 4 After drying, filtering, and rotary evaporation to remove dichloromethane, a light orange crude product was obtained. Subsequently, it was recrystallized with acetonitrile and dried in a vacuum oven at 60° C. for 6 hours to obtain 3.23 g of a refined light ...
Embodiment 3
[0063] Synthetic experiments were carried out in an oil bath. In a 250mL three-neck flask, completely dissolve 5.0g (10.46mmol) Rhodamine B in 180mL 40°C absolute ethanol, then quickly add 12.56g (209mmol) ethylenediamine liquid, slowly raise the oil bath to 85°C, The reaction was refluxed for 24 hours until the solution changed from fluorescent red to light yellow. After the reaction was complete, the solvent was removed by rotary evaporation to obtain a solid mixture, which was then extracted with 100 mL of dichloromethane and 100 mL of water, and the organic layer was repeatedly washed with deionized water (100 mL) five times to remove residual rhodamine B. Liquid separation to obtain an organic layer, anhydrous MgSO 4 After drying, filtering, and rotary evaporation to remove dichloromethane, a light orange crude product was obtained. Subsequently, it was recrystallized with acetonitrile and dried in a vacuum oven at 60° C. for 6 hours to obtain 3.23 g of a refined light ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com