C22orf26 gene and application of expression product of C22orf26 gene to preparing Parkinson diagnosis and treatment preparation
A C22ORF26, 1.C22ORF26 technology, applied in gene therapy, biological testing, biochemical equipment and methods, etc., can solve problems that cannot meet clinical needs, and achieve good application prospects, good stability, and fast response effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 High-throughput sequencing and analysis
[0031] The peripheral blood samples of 15 Parkinson's patients and 9 healthy people were collected for RNA extraction. After RNA extraction, agarose gel electrophoresis was performed. From the electrophoresis results, it can be preliminarily judged whether the quality of the extracted RNA samples is qualified or not, and whether they can be used for further transcriptome analysis. Then, the extraction of RNA samples was detected by NanoDrop1000 spectrophotometer, and the sample requirements for RNA-seq sequencing: OD260 / OD280 was 1.8-2.2.
[0032] The sequencing platform is the HiSeq2500 high-throughput sequencing platform of Illumina, which performs high-throughput transcriptome deep sequencing. After sequencing, we use Fast-QC (http: / / www.bioinformatics.babraham.ac.uk / projects / fastqc / ) software Overall assessment of the quality of sequencing data, including base quality value distribution, quality value position dis...
Embodiment 2
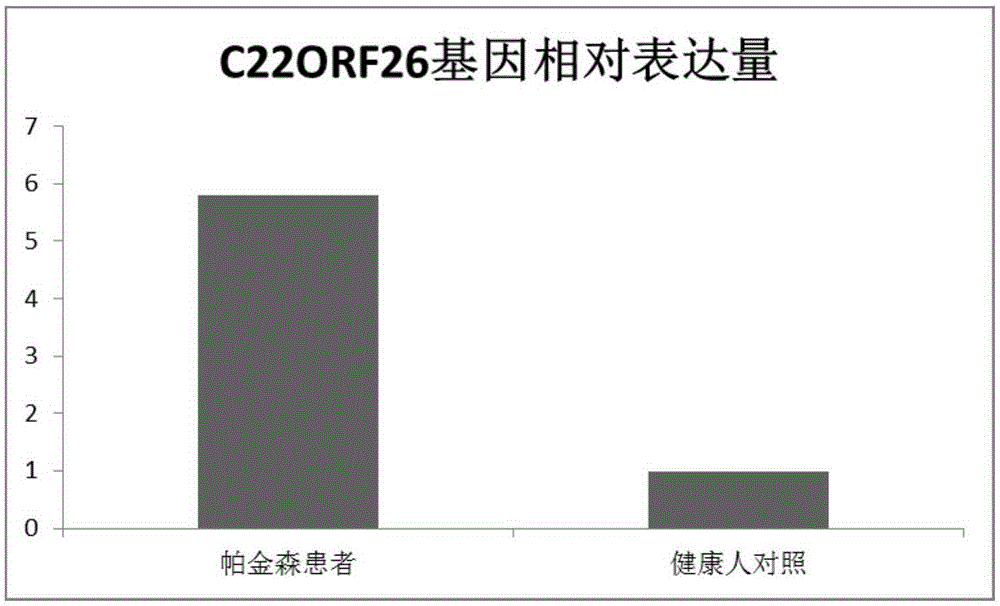
[0033] Example 2 C22ORF26 Gene Expression in Peripheral Blood of Parkinson's Patients and Peripheral Blood of Healthy People
[0034] 1. Materials and methods
[0035] 1. Materials
[0036] The peripheral blood of 135 cases of Parkinson's patients and 33 cases of healthy people were collected, grouped and numbered.
[0037] 2. Method
[0038] 2.1 Extraction of total RNA from peripheral blood of Parkinson's and healthy people
[0039] use Reagent was used to extract RNA from samples, and the experimental operation was performed according to the product instructions.
[0040] Judgment criteria for RNA quality: the OD260 / OD280 value of the RNA sample is between 1.7 and 2.2; the electrophoretic pattern of total RNA has clear 28S and 18S bands; difference.
[0041] 2.2 Synthesis of cDNA by reverse transcription
[0042] use IIIReverseTranscriptase (invitrogen, Cat. No. 18080-044) was used for cDNA reverse transcription, and the experimental operation was carried out accor...
Embodiment 3
[0062] The cultivation of embodiment 3 cell line SN4741
[0063] 1. Materials
[0064] SN4741 cells were donated by the Fourth Military Medical University.
[0065] 2. Experimental method
[0066] cell culture
[0067] First, prepare a special medium for SN cells: add FBS54ml, D-glucose 16.2ml, L-glutamine 3.8ml and penicillin / streptomycin double antibody to 500ml DMEM (4.5g / LD-glucose). Then take out the frozen SN4741 cells from liquid nitrogen, put them in a 37°C water bath and thaw quickly within 1 min, suck out the cell suspension, transfer it into a centrifuge tube with the prepared medium, mix gently and pipette, 1200rpm Centrifuge for 5 minutes, remove the supernatant, continue to blow gently and evenly to form a cell suspension, then transfer to a culture dish, at 33 ° C, 5% CO 2 Cultured in a constant temperature incubator with 100% humidity. When the cells are attached to the wall and the density reaches 75-85%, they can be subcultured. First remove the original...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com