Kit for activating ovarian cancer specific immunity response
An immune response and kit technology, applied in the field of kits for enhancing anti-cancer immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
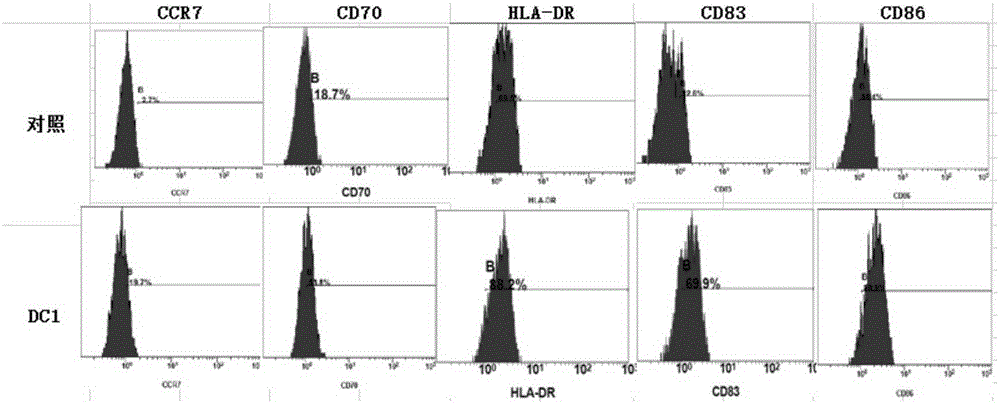
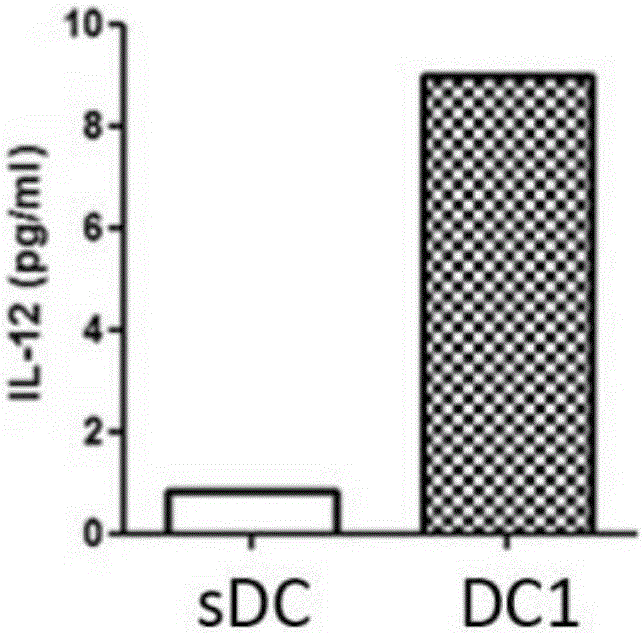
[0032] Embodiment 1 (cell culture supernatant is T cell culture supernatant)
[0033] When using the kit of the present invention to activate the specific immune response of ovarian cancer patients, the preparation of type 1 polarized dendritic cells (type1 polarized dendritic cells, DC1) derived from adult peripheral blood is firstly performed. Peripheral blood mononuclear cells were isolated according to the method described by Jonuleit et al. (GeneTher. 200310(3)). First, peripheral blood mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation, and plasma was collected for subsequent culture. By density 3.0×10 6 / ml, the cells were inoculated into T75 culture flasks, and 10% plasma was added to the GGT551 medium, placed at 37.0°C, saturated humidity, 5% CO 2 cultivated in the environment. After culturing for 3 hours, the suspended lymphocytes were washed away, and DMEM / F12 medium containing GM-CSF and IL-4 1000 IU / ml and 10% plasma was added. ...
Embodiment 2
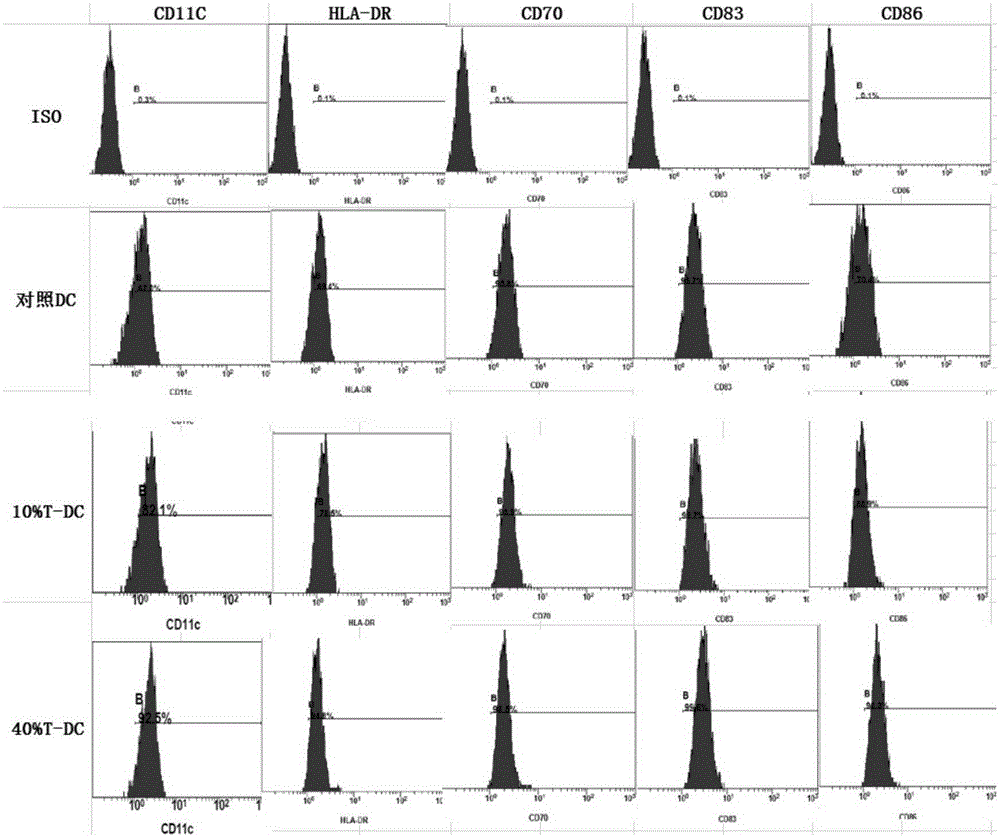
[0038] Embodiment two (cell culture supernatant is NK cell and K562 cell co-culture supernatant)
[0039] Peripheral blood mononuclear cells were isolated according to the method described by Jonuleit et al. (GeneTher. 200310(3)). First, peripheral blood mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation, and plasma was collected for subsequent culture. IL-2 (2000U / ml) and IL15 (2ng / ml) were added. Doubling the medium every two to three days, and supplementing the factors in equal amounts. Collected on the twelfth day. After the induction is completed, NK cells are treated by 1-3×10 6 / ml was inoculated into medium RPMI1640, IFNa (1000UI / ml) was added and 0.5-1.5×10 5 Inoculate K562 cells (can be replaced with K562-NK cells) at a density of / ml for mixed culture, collect the medium supernatant during 24-48 hours of activation, filter and freeze at -80 degrees or directly use it for the preparation of DC1 cells. Peripheral blood mononuclear ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com