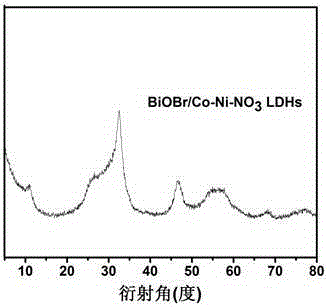
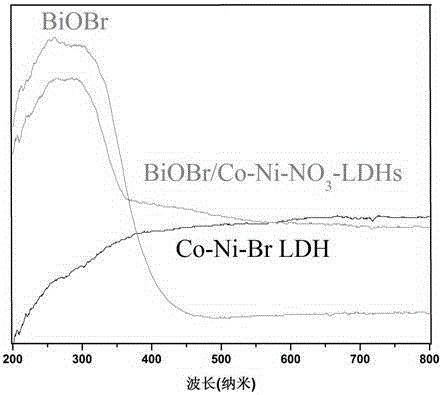
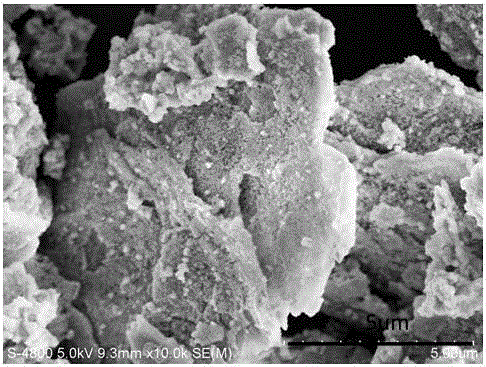
Preparation method of nano-material with bismuth oxybromide loaded on cobalt nickel hydrotalcite surface
A surface loading, bismuth oxybromide technology, applied in chemical instruments and methods, water pollutants, other chemical processes, etc., to achieve the effect of simple and easy operation of the experimental process, good prospects for development, and high catalytic repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Cobalt nickel hydrotalcite surface supported bismuth oxybromide (BiOBr / Co-Ni-NO 3 LDHs) nano photocatalytic preparation method, comprising the following steps:
[0026] (1) At room temperature, add cobalt chloride, nickel chloride and hexamethylenetetramine with a molar ratio of 2:1:18 into ultrapure water in sequence, dissolve them fully and put them into a hydrothermal reaction kettle. ℃ for 5 hours, after cooling, the product was separated by filtration, cleaned with deionized water and ethanol, and finally dried at room temperature to obtain cobalt-nickel hydroxide;
[0027] (2) At room temperature, weigh 0.372g of cobalt-nickel hydroxide and add it to an eggplant-shaped bottle filled with 200mL of acetonitrile and 13.34mmol of bromine water. Do not leak air, and keep stirring for 24 hours, then centrifuge the product, wash it with a large amount of ethanol, and finally dry it at room temperature to obtain Co-Ni-BrLDH;
[0028] (3) Dissolve bismuth nitrate and man...
Embodiment 1
[0032] At room temperature, weigh 0.9517g of cobalt chloride hexahydrate, 0.4754g of nickel chloride hexahydrate, and 5.0468g of hexamethylenetetramine into 400mL of ultrapure water in sequence, and stir until completely dissolved. It was equally divided into 5 parts and put into a 100mL hydrothermal reaction kettle, and reacted in an oven at 95°C for 5h. After cooling, the product was filtered, washed three times with deionized water and ethanol, and dried at room temperature to obtain Co-Ni hydroxide.
Embodiment 2
[0034] At room temperature, measure 0.69mL of bromine water and dissolve it in an eggplant-shaped bottle, then add 200mL of acetonitrile and 0.372g of Co-Ni hydroxide sample in sequence, and wrap the mouth of the bottle tightly with plastic wrap. Then pass into N 2 15min, and continued stirring for 24h. The product was separated by centrifugation, washed with a large amount of ethanol, and dried at room temperature to obtain Co-Ni-BrLDH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com