Synthesis method of metaldehyde
A synthetic method, metaldehyde technology, applied in the direction of organic chemistry, etc., can solve the problems of low product yield and high energy consumption, and achieve the effects of mild reaction conditions, easy industrialization, and increased selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The first embodiment of the present invention provides a method for synthesizing metaldehyde. The synthesis method includes catalyst preparation, polymerization reaction and depolymerization reaction.
[0028] Catalyst preparation: Add 4 g of furan hydrobromide under stirring with 6 g of hydrobromic acid (49%), stir and dissolve for later use.
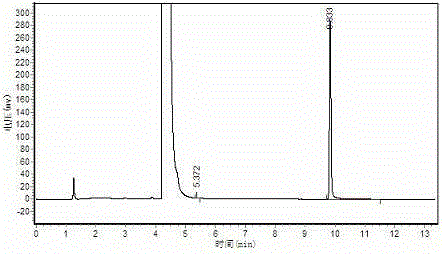
[0029] Polymerization: Stir 200ml of acetaldehyde down to -15°C, add 0.6g of the above-mentioned catalyst dropwise to the acetaldehyde, control the rate of addition, and ensure the temperature of the reaction solution. Heat and stir for 1 hour, then filter, wash the solid phase with water, and dry to obtain 20.5 g of white crystal metaldehyde, with a yield of 13.1%. Content>99%. The results of gas chromatography were as attached figure 1 , peak time: 5.372min, 9.833min; Area%: 0.153, 99.847.
[0030] Depolymerization reaction: Take 160ml of the filtrate, add 5 grams of phosphoric acid: sulfuric acid (V:V=50:50) composite c...
Embodiment 2
[0032] The second embodiment of the present invention provides a method for synthesizing metaldehyde. The synthesis method includes catalyst preparation, polymerization reaction and depolymerization reaction.
[0033] Catalyst preparation: Add 4 g of piperazine hydrobromide under stirring with 6 g of hydrobromic acid (49%), stir and dissolve for later use.
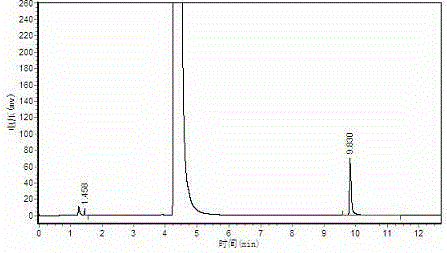
[0034] Polymerization: Stir 200ml of acetaldehyde down to -15°C, add 0.6g of the prepared catalyst dropwise to the acetaldehyde, control the rate of addition, and ensure the temperature of the reaction solution, the highest is not higher than 10°C, after the catalyst is added, it will be at -5-5 Stir at ℃ for 1 hour, then filter, wash the solid phase with water, and dry to obtain 22.3 g of white crystal metaldehyde, with a yield of 14.3% and a content of >99%. The results of gas chromatography were as attached figure 2 , peak time: 1.458min, 9.830min; Area%: 0.229, 99.771.
[0035] Depolymerization reaction: Add 5 gra...
Embodiment 3
[0037] The third embodiment of the present invention provides a method for synthesizing metaldehyde. The synthesis method includes catalyst preparation, polymerization reaction and depolymerization reaction.
[0038] Catalyst preparation: Add 4 g of pyridine hydrobromide under stirring with 6 g of hydrobromic acid (49%), stir and dissolve for later use.
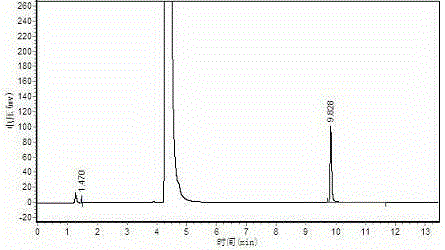
[0039] Polymerization: Stir 200ml of acetaldehyde down to -15°C, add 0.6g of the prepared catalyst dropwise to the acetaldehyde, control the rate of addition, and ensure the temperature of the reaction solution, the highest is not higher than 10°C, after the catalyst is added, it will be at -5-5 Stir at ℃ for 1 hour, then filter, wash the solid phase with water, and dry to obtain 23.5 g of white crystal metaldehyde, with a yield of 15.1% and a content of >99%. Gas chromatography test results like image 3 , peak time: 1.470min, 9.828min; Area%: 0.268, 99.732.
[0040] Polymerization reaction: add 5 grams of phosphoric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com