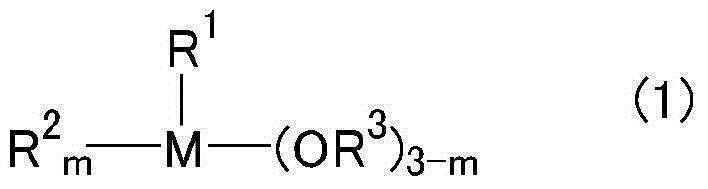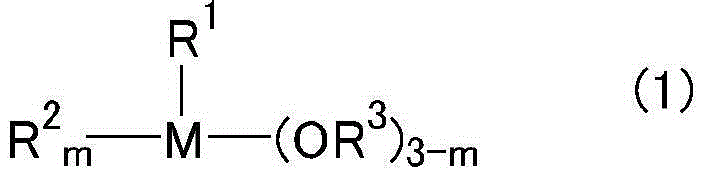Photosensitive resin composition, photospacer, protective film for color filters, and protective film or insulating film of touch panel
A technology of photosensitive resin and composition, which is applied in the field of photosensitive resin composition, can solve the problems of reduced productivity, long developing time, and inability to obtain photosensitive resin composition, etc., and achieve excellent adhesion, high developability, excellent Effect of elastic recovery properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0184] 60 parts of styrene, 20 parts of methyl methacrylate, 20 parts of methacrylic acid and diethylene glycol dimethyl ether 217 share. After the gas phase in the system was replaced with nitrogen, a solution 36 of 6 parts of 2,2'-azobis(2,4-dimethylvaleronitrile) dissolved in 30 parts of diethylene glycol dimethyl ether was added. parts, heated to 90°C, and further reacted at the same temperature for 4 hours. Further, 15 parts of glycidyl methacrylate and 1 part of triethylamine were added to the obtained solution, and reacted at 90° C. for 6 hours to obtain 30% diethylene glycol dimethyl of hydrophilic vinyl resin (A-1). base ether solution.
[0185] The solid conversion acid value of the hydrophilic vinyl resin was 131.5. Mn based on GPC was 4,000. In addition, the SP value of the hydrophilic vinyl resin was 10.8, and the HLB value was 7.1.
manufacture example 2
[0187] 90 parts of styrene, 10 parts of methyl methacrylate, 30 parts of methacrylic acid, and 327 parts of propylene glycol monomethyl ether acetate were placed in a glass flask equipped with a heating and cooling / stirring device, a reflux cooling tube, and a nitrogen introduction tube. share. After replacing the gas phase in the system with nitrogen, add a solution 38 of 8 parts of 2,2'-azobis(2,4-dimethylvaleronitrile) dissolved in 30 parts of propylene glycol monomethyl ether acetate. parts, heated to 90°C, and further reacted at the same temperature for 4 hours. Further, 15 parts of glycidyl methacrylate and 1 part of triethylamine were added to the obtained solution, and reacted at 90° C. for 6 hours to obtain 30% propylene glycol monomethyl ether ethyl alcohol of hydrophilic vinyl resin (A-2). acid ester solution.
[0188] The solid conversion acid value of the hydrophilic vinyl resin was 96.1. Mn based on GPC was 4,500. In addition, the SP value of the hydrophilic ...
manufacture example 3
[0190] Put cresol novolak type epoxy resin "EOCN-102S" (Nippon Kayaku Co., Ltd. epoxy equivalent weight 200 ) and 145 parts of propylene glycol monomethyl ether acetate were heated to 90°C to dissolve them uniformly. Next, 76 parts of acrylic acid, 2 parts of triphenylphosphine, and 0.2 part of p-methoxyphenol were added, and it was made to react at 90 degreeC for 10 hours.
[0191]91 parts of tetrahydrophthalic anhydrides were further added to the reactant, further reacted at 90° C. for 5 hours, and then diluted with propylene glycol monomethyl ether acetate so that the content of the hydrophilic epoxy resin became 60%, A 60% propylene glycol monomethyl ether acetate solution of the hydrophilic epoxy resin (A-3) was obtained.
[0192] The solid conversion acid value of the hydrophilic epoxy resin was 88.4. Mn based on GPC was 2,200. In addition, the SP value of the hydrophilic epoxy resin was 11.3, and the HLB value was 9.8.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com