A kind of alkali production process
A production process and cathode chamber technology, applied in the field of alkali production process, can solve the problems of electrolyte resistivity reduction, waste, anode conductivity reduction, etc., so as to prevent alkali from re-reacting, reduce waste of electric energy, and improve electrolysis rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
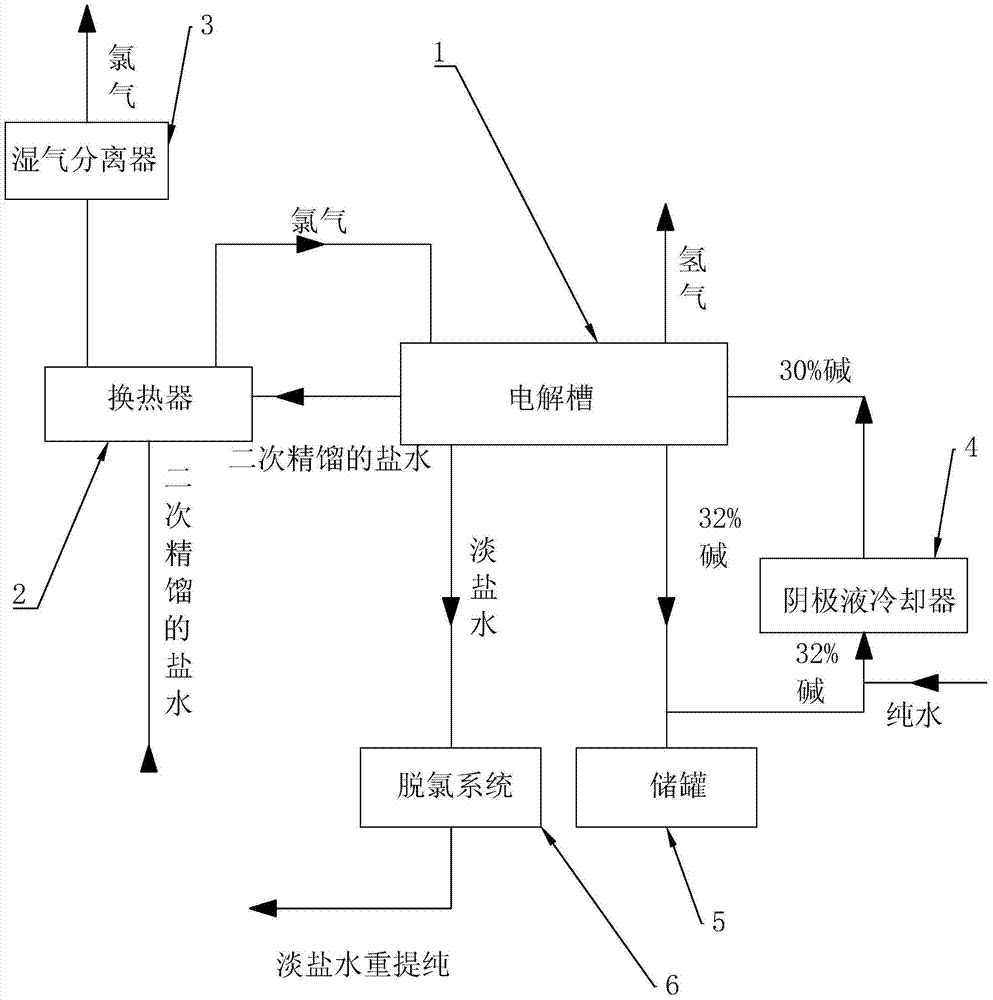
[0017] refer to figure 1 The embodiments of the present invention will be further described.
[0018] The present invention is a kind of production process of alkali, and alkali described here mainly refers to the sodium hydroxide solution that mass fraction is 32%, and its step comprises, the brine of 18.1~18.9% secondary rectification, with 28m 3 The flow rate of / hr passes through the cold fluid pipeline of the heat exchanger 2, enters the anode chamber of the electrolytic cell 1 after being preheated, and the temperature reached by the preheating is 55°C. The cathode chamber is connected with a mixture of 32±0.5% alkali and pure water produced by the electrolysis of the cathode chamber. The concentration after mixing is 30±0.5%, and the flow of catholyte is controlled at 14m 3 / hr. After that, start to gradually increase the current in the solution, and when the current reaches 5KA, start to gradually add acid to the anode chamber, adjust the pH to 4 at first, and then c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com