Improved industrialization technology for preparing Vildagliptin
A process and technology of acylation reaction, applied in the field of medicine, to achieve good tolerance, shorten the reaction time, and improve the effect of the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be described in more detail below with examples, but it cannot be considered that the present invention is limited to this example.
[0022] (1) Acylation reaction
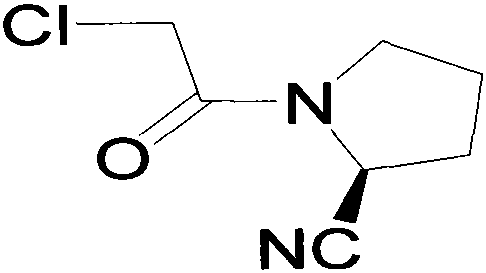
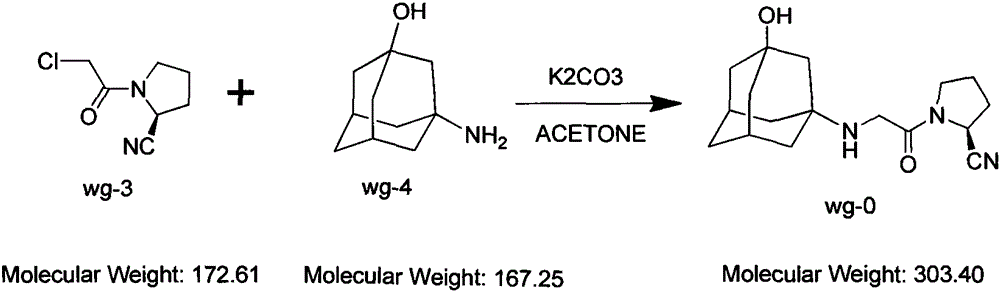
[0023] Add wg-11400g and tetrahydrofuran 40g into the reaction kettle and stir to dissolve at room temperature. After stirring for 15 minutes, add 2000g of triethylamine dropwise (the temperature does not change much during the dropwise addition), and cool down to -2°C to -10°C after dropping. Add 1700g of chloroacetyl chloride dropwise at temperature -10°C~-5°C (white fumes are generated when dropping); after dropping, keep warm for 1 hour (the residual wg-1 detected by HPLC is less than 5%; after the reaction, the reaction solution is light purple mixed suspension). The molar yield is between 60% and 78%.
[0024] (2) Dehydration reaction
[0025] Cool the wg-2 solution obtained in the previous step to below 0°C, and add TFAA5200g dropwise at 0°C~-5°C (white smoke is generate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com