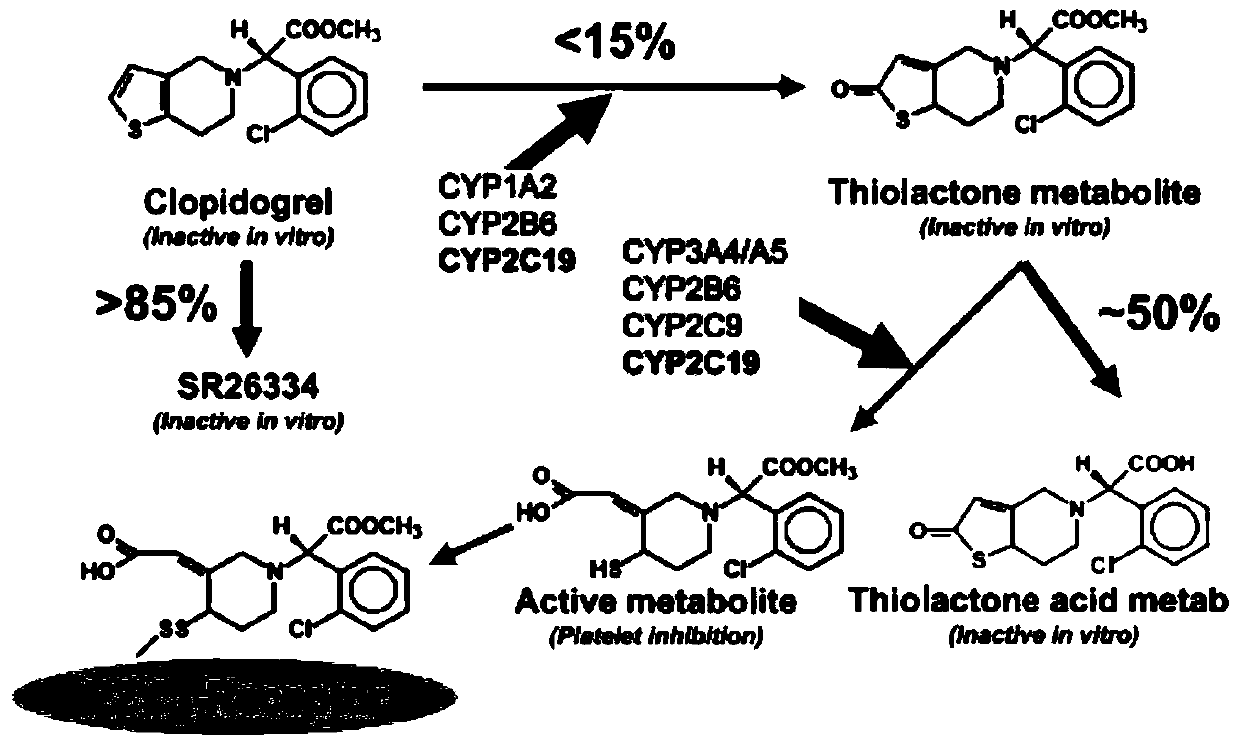
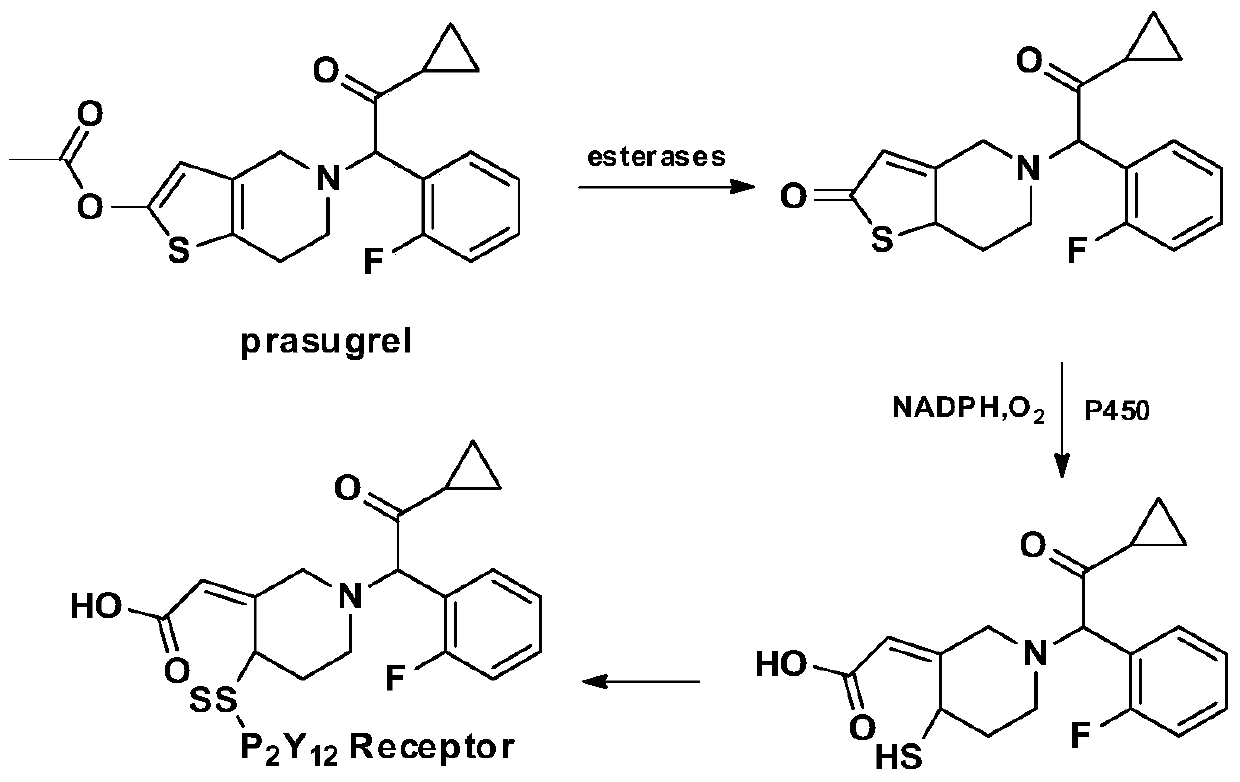
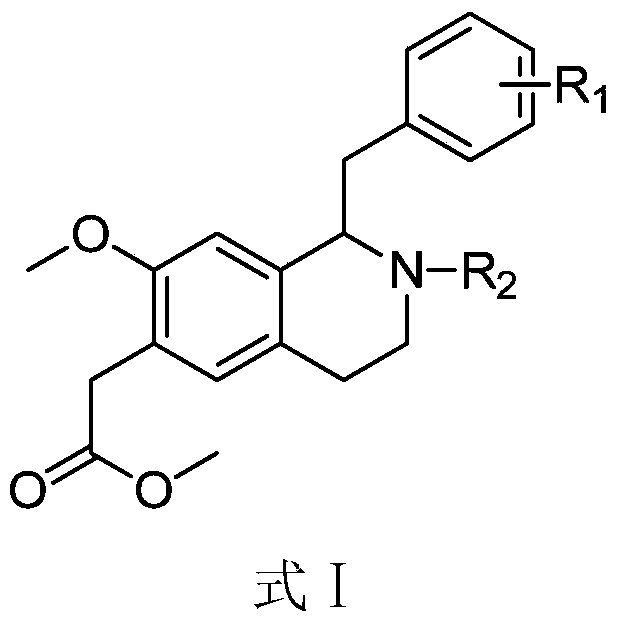
A kind of 1-benzyl-6-methoxycarbonylmethyl tetrahydroisoquinoline derivative, its preparation method and its anti-platelet aggregation application
A technology of methoxycarbonyltetrahydroisoquinoline and methoxycarbonylmethyl, which is applied in the field of drug synthesis to achieve the effects of low cost, simple and easy preparation method, and correct structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of 2-[(1,1'-biphenyl)-4-yl]-N-(4-methoxyphenethyl)acetamide (compound 9)
[0042] Weigh 1 mmol of p-methoxyphenethylamine hydrochloride, 1.0-1.2 mmol of felbinac, dissolve the raw materials in 5 mL of acetonitrile in a 25 mL single-necked round bottom flask, add 4.2 mmol of triethylamine, and stir magnetically in a water bath at 25 °C. After 5 minutes, 0.4930 g (1.3 mmol) of peptide coupling agent HBTU was added, and the reaction was complete in 15 to 60 minutes. Acetonitrile was removed by rotary evaporation under reduced pressure to obtain a yellow solid, which was dissolved in 50 mL of dichloromethane, washed with deionized water (50 mL×3), washed with 50 mL of saturated brine, and the dichloromethane layer was dried with anhydrous sodium sulfate, suction filtered, and spun under reduced pressure. The solvent was evaporated to give a white solid. Column chromatography separation (dichloromethane:petroleum ether:ethyl acetate=100:25:4) gave 0.33...
Embodiment 2
[0043] Example 2 2-[(1,1'-biphenyl)-4-yl]-N-(3-phenylmercaptomethoxycarbonylmethyl-4-methoxyphenethyl)acetamide (Compound 10) preparation of
[0044] (1) Preparation of methyl 2-phenylthioglycolate (compound 7). Measure 2054 μL (20.0 mmol) of thiophenol into a 100 mL three-neck flask, add 30 mL of toluene, cut 0.5060 g (22.0 mmol) of sodium metal into small pieces and add it to the bottle, reflux at 110 ° C, and monitor the progress of the reaction by TLC. After the reaction is complete, add 20.0 to 30.0 mmol of methyl chloroacetate dropwise with a constant pressure dropping funnel, monitor the reaction by TLC, and the reaction is complete after 1 to 2 hours. Add 70 mL of ethyl acetate, wash with 100 mL of deionized water three times, dry the organic phase with anhydrous sodium sulfate, filter with suction, and remove the solvent by rotary evaporation under reduced pressure to obtain a light yellow oily liquid 1. Column chromatography (ethyl acetate:petroleum ether=1:40) gav...
Embodiment 3
[0047] Example 3 Preparation of 2-[(1,1'-biphenyl)-4-yl]-N-(3-methoxycarbonylmethyl-4-methoxyphenethyl)acetamide (compound 11)
[0048] Weigh 3.9813g (7.6mmol) of compound 10 into a 250mL single-necked flask, and dissolve in 80mL of ethanol. Wash the Raney nickel with ethanol to remove water, add 11.4g of Raney nickel, replace with hydrogen 3 times, put the hydrogen in a water bath at 25°C and stir vigorously with magnetic force, and the reaction is complete in 2 to 4 hours. The reaction solution was suction-filtered with diatomaceous earth, the filter cake was washed with dichloromethane, and the filtrate was rotary evaporated under reduced pressure to remove the solvent to obtain 3.0500 g of a white solid with a yield of 97%. 1 H NMR (CDCl 3 ,400MHz)δ:7.59(d,J=7.2Hz,2H,H-2",6"),7.55(d,J=8.0Hz,2H,H-4,8),7.44(dd,J 1 =7.2Hz,J 2 =7.2Hz, 2H, H-3”, 5”), 7.35(t, 1H, J=7.2Hz, 1H, H-4”), 7.26(d, J=8.0Hz, 2H, H-5,7 ),6.90(d,J=1.4Hz,H-4'),6.89(dd,1H,J 1 =1.4Hz,J 2 =8.0Hz, H-8'), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com