Zinc-based heat stabilizer for polyvinyl chloride, composition with same and application of zinc-based heat stabilizer for polyvinyl chloride
A polyvinyl chloride, zinc-based heat technology, applied in the field of PVC heat stabilizers, can solve the problems of vicious degradation of PVC samples, reduced HCl removal rate, poor long-term thermal stability, etc., and achieves low production cost, simple process operation, comprehensive Good thermal stabilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
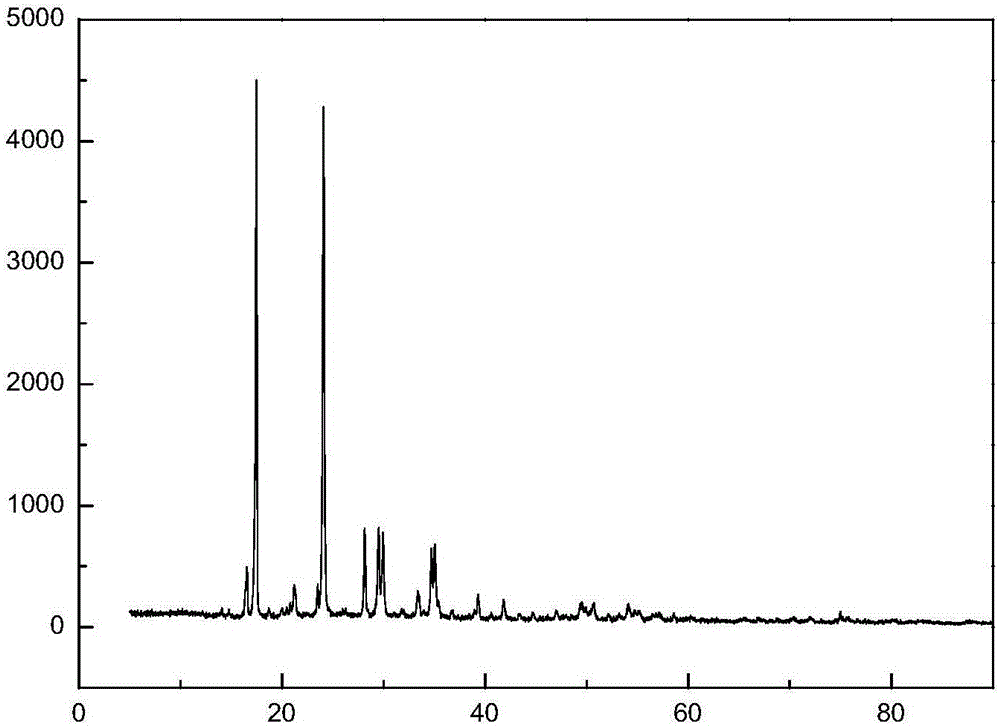
[0020] Biuret-zinc synthesis reference (Zang Qing, Wang Meiling, Zhong Guoqing. Synthesis and characterization of biuret-based complexes. Fine Chemical Industry, 2015,32(7):768-771.) and optimize the method : Dissolve 0.02mol of biuret in 150mL of ethanol at 60°C, add 0.01mol of anhydrous zinc acetate under stirring, keep the temperature and stirring state unchanged, react for 6h, filter and separate to obtain the crude product, and then wash with ethanol and deionized water Twice, dry at 60°C for 3 hours to obtain a white powder, which is recorded as biuret-zinc. ICP analysis zinc content is 22%, infrared absorption spectrum data (cm -1 KBr tablet): 3778(w), 3571(w), 3405(m), 3351(w), 3225(m), 2925(w), 2359(w), 1711(s), 1565(m), 1472(w), 1411(m), 1219(m), 1106(s), 779(m), 618(w), 552(w), 432(w), see XRD test results figure 1 .
Embodiment 2
[0025] Thiourea-zinc synthesis: Dissolve 0.03mol thiourea in 150mL ethanol, add 0.01mol anhydrous zinc acetate under stirring at room temperature, keep the temperature and stirring state unchanged, react for 4 hours, filter and separate to obtain the crude product, and then wash with ethanol Twice, dry at 60°C for 3 hours to obtain a white powder, which is recorded as biuret-zinc. ICP analysis zinc content is 24%, infrared absorption spectrum data (cm -1KBr tablet): 3379(m), 3305(m), 3192(m), 3099(m), 3052(m), 2752(w), 1646(s), 1592(s), 1505(m), 1398(s), 1332(m), 1132(w), 1035(w), 933(w), 779(m), 672(m), 605(w), 519(m), 479(m), 412 (m); proton nuclear magnetic resonance spectrum analysis result is: 1 HNMR (400MHz, DMSO) δ7.32 (br, 8H), 1.78 (s, 6H), XRD test results see figure 2 .
[0026] Mixing formula: 100g PVC resin, 20g dioctyl phthalate and 3g thiourea-zinc. Preparation, test method and data list are the same as application example 1.
Embodiment 3
[0028] Synthesis of thiourea dioxide-zinc: Dissolve 0.02mol thiourea dioxide in 150mL 55°C deionized water, add 0.01mol anhydrous zinc acetate under stirring, keep the temperature and stirring state unchanged, react for 6h, and vacuum rotary evaporate 100mL solvent at 70°C, The crude product was isolated by filtration, washed twice with deionized water, and dried at 70°C for 3 hours to obtain a white powder, which was designated as thiourea-zinc dioxide. ICP analysis zinc content is 23%, infrared absorption spectrum data (cm -1 KBr tablet): 3364(s), 3251(m), 3171(s), 2964(w), 1638(s), 1565(s), 1498(w), 1405(m), 1279(w), 1219(m), 1125(m), 1005(m), 806(m), 650(w), 605(w), 460(w), see XRD test results image 3 .
[0029] Mixing formula: 100g PVC resin, 50g dioctyl phthalate and 1g thiourea-zinc dioxide. Preparation, test method and data list are the same as application example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com