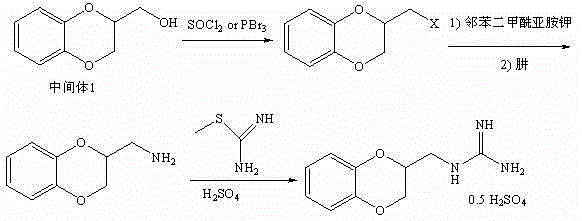
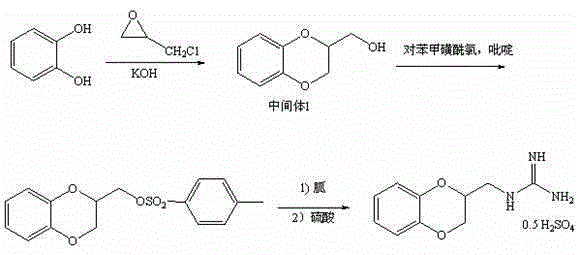
Guanoxan sulfate synthesis method
A technology of guanidine sulfate production and synthesis method, which is applied in the field of organic drug synthesis, can solve the problems of high toxicity of 1,2-epichlorohydrin, influence the market competitiveness of products, and many synthesis steps, and achieves low toxicity and short synthesis route. , the effect of simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of guanidine sulfate synthetic method, it comprises the steps:
[0024] 1) Synthesis of 2-hydroxymethyl-1,4-benzodioxane:
[0025] Add 20g (182mmol) of ortho-catechol, 80g of water and 39.6g (990mmol) of sodium hydroxide into the reaction bottle, protect it with nitrogen, heat (about 70-80°C) and stir to dissolve, then add 30ml of toluene, stir slowly 29.5 g (200 mmol) of 1,2,3-trichloropropane was added dropwise, and after the dropwise addition was completed, the temperature was raised and refluxed for reaction for 3.5 hours. After the reaction, cool down to below 15°C, separate the organic layer, extract the water layer twice with toluene (20ml*2), combine the toluene layer, wash with water, evaporate the toluene under reduced pressure, add 50ml of water to the residue, heat to dissolve and cool down to 15°C Below ℃, crystals were precipitated, filtered, and dried to obtain 25.0 g of solid 2-hydroxymethyl-1,4-benzodioxane, with a yield of 82%.
[0026] 2) Syn...
Embodiment 2
[0031] A kind of guanidine sulfate synthetic method, it comprises the steps:
[0032] 1) Synthesis of 2-hydroxymethyl-1,4-benzodioxane:
[0033] Add 20g (182mmol) of ortho-catechol, 80g of water and 51g (910mmol) of potassium hydroxide into the reaction bottle, protect with nitrogen, heat (about 70-80°C) and stir to dissolve, then add 30ml of toluene, slowly drop under stirring 29.5 g (200 mmol) of 1,2,3-trichloropropane was added, and after the dropwise addition was completed, the temperature was raised to reflux for 3.5 hours. After the reaction, cool down to below 15°C, separate the organic layer, extract the water layer twice with toluene (20ml*2), combine the toluene layer, wash with water, evaporate the toluene under reduced pressure, add 50ml of water to the residue, heat to dissolve and cool down to 15°C Below ℃, crystals were precipitated, filtered, and dried to obtain 25.7 g of solid 2-hydroxymethyl-1,4-benzodioxane, with a yield of 85%.
[0034] 2) Synthesis of 2-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com