Method for preparing 1-cyclohexenylacetic acid
A technology of cyclohexenyl acetic acid and ammonium acetate, which is applied in the preparation of carboxylic acid nitrile, the preparation of organic compounds, the preparation of nitrile, etc., can solve the problems of low product purity and low yield, and achieve stable reaction, improved yield, The effect of avoiding safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
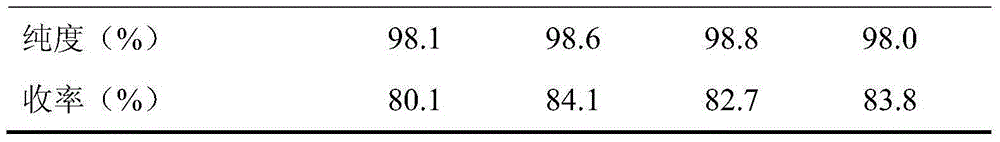
[0018] Add cyclohexanone, n-hexane, and ammonium acetate into a four-neck flask equipped with a stirrer, reflux condenser, and oil-water separator, stir and heat to 90°C, then drop cyanoacetic acid, and control the dropping time to 0.5h, wherein, The molar ratio of cyclohexanone and cyanoacetic acid is 1:0.8, the quality of n-hexane is 1 times of the total mass of cyclohexanone and cyanoacetic acid, the quality of ammonium acetate is 1.5% of the total mass of cyclohexanone and cyanoacetic acid, A dehydration reaction occurs, the temperature of the dehydration reaction is controlled at 120° C., and the dehydration time is 3 hours to obtain the intermediate of cycloalkenylene cyanoacetic acid.
[0019] Weigh acetic acid whose quality is 2 times of the intermediate of cycloalkenyl cyanoacetic acid, dissolve the intermediate of cycloalkenyl cyanoacetic acid to make a solution and put it into a reaction kettle after heating, and weigh 1% ammonium acetate accounting for the total mas...
Embodiment 2
[0022] Add cyclohexanone, n-hexane, and ammonium acetate into a four-neck flask equipped with a stirrer, reflux condenser, and oil-water separator, stir and heat to 95°C, then drop in cyanoacetic acid, and control the dropping time to 0.6h, wherein, The molar ratio of cyclohexanone and cyanoacetic acid is 1:1, the quality of n-hexane is 1.5 times of the total mass of cyclohexanone and cyanoacetic acid, the quality of ammonium acetate is 2% of the total mass of cyclohexanone and cyanoacetic acid, A dehydration reaction occurs, the temperature of the dehydration reaction is controlled at 140° C., and the dehydration time is 4 hours to obtain the intermediate of cycloalkenylene cyanoacetic acid.
[0023] Take by weighing acetic acid whose quality is 2.5 times of the intermediate of cycloalkenyl cyanoacetic acid, dissolve the intermediate of cycloalkenyl cyanoacetic acid to make a solution and put it into a reaction kettle after heating, and weigh 2% ammonium acetate that accounts ...
Embodiment 3
[0026] Add cyclohexanone, n-hexane, and ammonium acetate into a four-neck flask equipped with a stirrer, reflux condenser, and oil-water separator, stir and heat to 90°C, then drop cyanoacetic acid, and control the dropping time to 0.5h, wherein, The molar ratio of cyclohexanone and cyanoacetic acid is 1:1, the quality of n-hexane is 2 times of the total mass of cyclohexanone and cyanoacetic acid, the quality of ammonium acetate is 2.5% of the total mass of cyclohexanone and cyanoacetic acid, A dehydration reaction occurs, the temperature of the dehydration reaction is controlled at 120° C., and the dehydration time is 3 hours to obtain the intermediate of cycloalkenylene cyanoacetic acid.
[0027] Weigh acetic acid whose quality is 3 times of the intermediate of cycloalkenyl cyanoacetic acid, dissolve the intermediate of cycloalkenyl cyanoacetic acid to make a solution, heat it, put it into a reaction kettle, and weigh 2% ammonium acetate of the total mass of the solution Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com