(S)-4-hydroxyl-2-oxo-1-pyrrolidine acetamide sustained release tablet and preparing method thereof
一种缓释片、羟丙甲基的技术,应用在医药配方、非有效成分的医用配制品、含有效成分的医用配制品等方向,能够解决降低药物使用方便性、患者使用不方便、危害患者健康等问题,达到减少药物不良反应、减少服用次数、提高安全性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A levoxiracetam slow-release tablet, prepared according to the following steps:
[0026] Chip composition
[0027]
[0028] Coating composition:
[0029]
[0030] Preparation process:
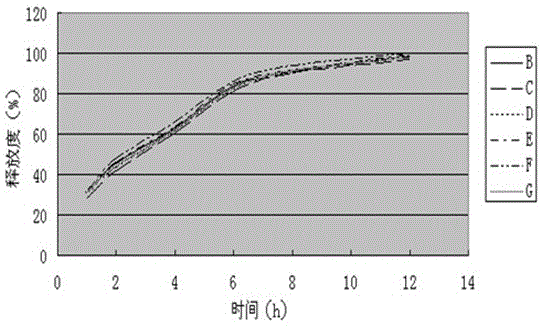
[0031] (1) Mix and pulverize levoxiracetam and the slow-release framework material into fine powder (the amount that passes through the No. 5 sieve and the No. 6 sieve that can pass through shall not be less than 95% of the total amount), and sieve;
[0032] (2) Add binder, mix and granulate (pass through 18 mesh sieve), place the prepared wet granules in a hot air oven, set the temperature at 40-60°C, dry until the moisture content of the granules is ≤3%, and granulate (pass through 18 mesh sieve), standby;
[0033] (3) Glidant, lubricant, anti-tack agent are pulverized through a 100-mesh sieve, added to the granulated granules, and mixed evenly;
[0034] (4) Tablet coating: adjust the tablet press, press the tablet, and pack the compressed sustained-release tablet with a mois...
Embodiment 2
[0052] A levoxiracetam slow-release tablet, prepared according to the following steps:
[0053] Chip composition
[0054]
[0055] Coating composition:
[0056]
[0057] Preparation process: prepared according to the preparation process of Example 1.
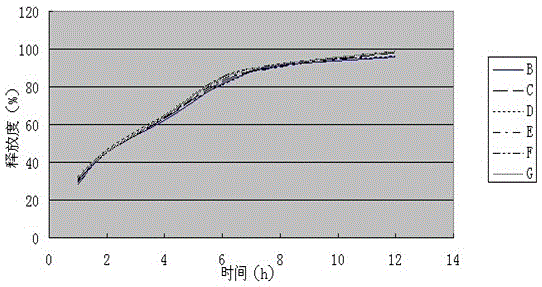
[0058] (1) Determination of release rate
[0059] Measure according to embodiment 1 release degree measuring method, the measurement result of its release degree is shown in Table 2, figure 2 (done six samples to measure).
[0060] Table 2 Sustained-release tablet sample release rate (%) of the present invention
[0061]
[0062] test results:
[0063] Appearance: Film-coated tablet with smooth surface.
[0064] Release: Levoxiracetam Sustained-release Tablets The main ingredient, Levo-Oxiracetam, is released slowly, which can meet the requirements of sustained-release tablets.
Embodiment 3
[0066] A levoxiracetam slow-release tablet, prepared according to the following steps:
[0067] Chip composition
[0068]
[0069] Coating composition:
[0070]
[0071] Preparation process: prepared according to the preparation process of Example 1.
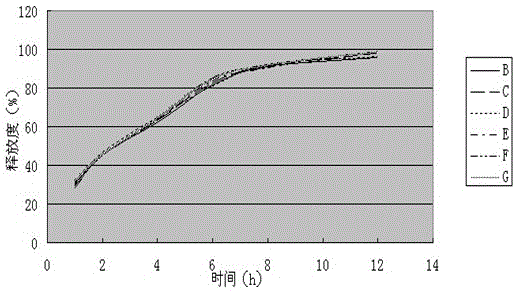
[0072] (1) Determination of release rate
[0073] Measure according to embodiment 1 release degree measuring method, the measurement result of its release degree is shown in Table 3, image 3 (done six samples to measure).
[0074] (1) The assay results of the release of the sustained-release tablet sample of the present invention are shown in Table 3, image 3 (done six samples to measure).
[0075] Table 3 Sustained-release tablet sample release rate (%) of the present invention
[0076]
[0077] test results:
[0078] Appearance: Film-coated tablet with smooth surface.
[0079] Release: Levoxiracetam Sustained-release Tablets The main ingredient, Levo-Oxiracetam, is released slowly, which can meet the require...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com