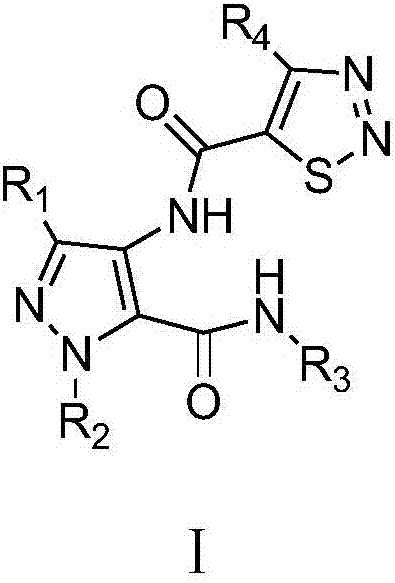
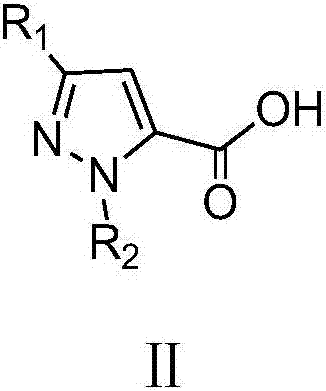
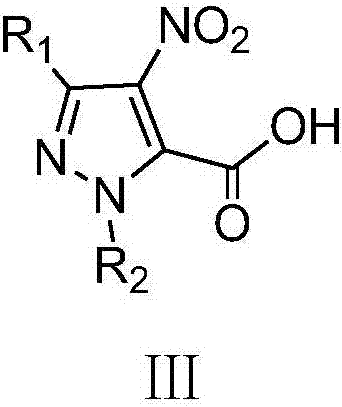
A class of pyrazole bisamide compounds containing 1,2,3-thiadiazole and its synthesis method and application
A technology of pyrazole bisamide and thiadiazole is applied in the field of pyrazole bisamide compounds and their synthesis, which can solve the problems of weak lipophilicity and strong hydrophilicity, achieve good armyworm killing activity and room for modification and modification. Large, novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 N-(3-ethyl-5-(methylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide
[0038] (1) 3-Ethyl-4-nitro-1H-pyrazole-5-carboxylic acid
[0039] In the 50mL there-necked flask, add 7.5mL (183.78mmol) fuming nitric acid, slowly add 8.4mL (170.57mmol) of fuming sulfuric acid dropwise under the ice bath, control the temperature of the system to be lower than 5°C during the dropwise addition, and add 3- Ethyl-1H-pyrazole-5-carboxylic acid 7.0g (50mmol), reacted at 60°C for 18h, cooled to room temperature, poured the reaction solution into 100g of ice, a white precipitate appeared, suction filtered, and dried to obtain 6.64g of white solid, Yield: 71.78%, m.p.150~152℃;
[0040] (2) N-methyl-3-ethyl-4-nitro-1H-pyrazole-5-carboxamide
[0041] 10g (54mmol) of 3-ethyl-4-nitro-1H-pyrazole-5-carboxylic acid and 51mL (702mmol) of thionyl chloride were added to a 100mL there-necked flask, heated to reflux for 4h, the reaction was completed, and the unreacted ...
Embodiment 2
[0050] Example 2 N-(3-ethyl-5-(ethylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide
[0051] (1) The preparation of 3-ethyl-4-nitro-1H-pyrazole-5-carboxylic acid refers to Example 1;
[0052] (2) Preparation of N,3-diethyl-4-nitro-1H-pyrazole-5-carboxamide Reference Example 1: white solid, yield: 41.01%, m.p.150~152°C. 1 H NMR (CDCl 3 ,ppm): 1.28~1.35(m, 6H), 2.99~3.07(q, J=7.4Hz, 2H), 3.52~3.61(m, 2H), 9.04(s, 1H), 12.20(s, 1H); MS:210.8[M-H] - ;
[0053] (3) The preparation of N,3-diethyl-4-amino-1H-pyrazole-5-carboxamide refers to Example 1;
[0054] (4) Refer to Example 1 for the preparation of 4-methyl-1,2,3-thiadiazole-5-formyl chloride;
[0055] (5) N-(3-ethyl-5-(ethylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-carboxamide The preparation of the reference Example 1: white solid, yield: 73.78%, m.p.196 ~ 197 ℃; 1 H NMR (CH 3 OH-d 4 ,ppm):1.18~1.23(t,J=7.2Hz,3H),1.24~1.29(t,J=7.6Hz,3H),2.71~2.78(q,J=7.6Hz,2H),2.94(s, 3H),3.39~3....
Embodiment 3
[0056] Example 3 N-(3-ethyl-5-(n-propylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-methyl amide
[0057] (1) The preparation of 3-ethyl-4-nitro-1H-pyrazole-5-carboxylic acid refers to Example 1;
[0058] (2) Reference Example 1 for the preparation of 3-ethyl-N-n-propyl-4-nitro-1H-pyrazole-5-carboxamide: white solid, yield: 73.00%, m.p.132~134°C; 1 H NMR (DMSO, ppm): 0.87~0.92 (t, J=7.4Hz, 3H), 1.20~1.25 (t, J=7.5Hz, 3H), 1.47~1.54 (m, 2H), 2.86~2.94 (q , J=7.5Hz, 2H), 3.19(m, 2H), 8.50(s, 1H); MS: 227.1[M+H] + ;
[0059] (3) The preparation of 3-ethyl-N-n-propyl-4-amino-1H-pyrazole-5-carboxamide refers to Example 1;
[0060] (4) Refer to Example 1 for the preparation of 4-methyl-1,2,3-thiadiazole-5-formyl chloride;
[0061] (5) N-(3-ethyl-5-(n-propylcarbamoyl)-1H-pyrazol-4-yl)-4-methyl-1,2,3-thiadiazole-5-methyl For the preparation of amide, refer to Example 1: white solid, yield: 93.83%, m.p.186~187°C; 1 H NMR (CH 3 OH-d 4 , ppm): 0.93~0.98 (t, J=7.4Hz, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com