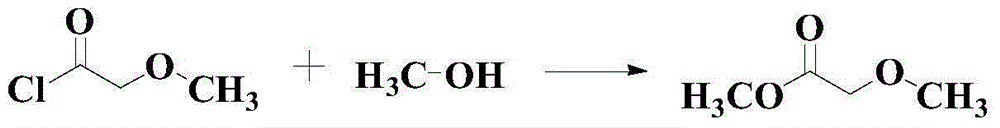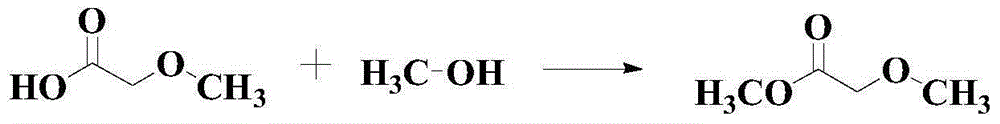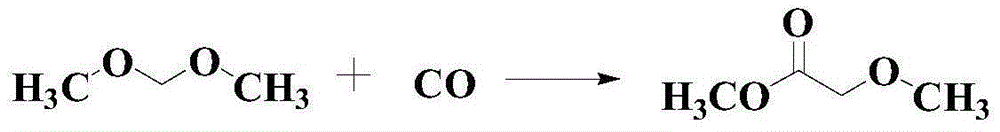Catalytic synthesis method for methyl methoxyacetate
A technology for the synthesis of methyl methoxyacetate and its synthesis method, which is applied in the field of catalytic synthesis of methyl methoxyacetate and the synthesis of ester compounds, which can solve the problem that the scope of application of substrates is not wide enough, and it is difficult to meet the requirements of synthesis of a large number of chemical products and functional materials. Demand and other issues, to achieve the effect of good industrial production potential and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0034] Preparation Example 1 of Solid Carrier Supported Catalyst: Preparation of Catalyst G1
[0035] S1: calcining carbon nanotubes at 800° C. for 15 minutes, and cooling naturally to obtain calcined carbon nanotubes;
[0036] S2: add the calcined carbon nanotubes and the active agent N,N,N',N'-tetramethyl-N,N'-disulfonic acid propylpropylenediamine bisulfate into an appropriate amount of organic solvent polyethylene glycol In alcohol (PEG-200), fully stirred at 70°C for 40 minutes, then stood still for 10 hours, filtered, and the filtered solid was fully washed with deionized water and dried completely to obtain the solid supported catalyst, named as G1;
[0037] Wherein, the mass ratio of the calcined carbon nanotubes to the active agent N,N,N',N'-tetramethyl-N,N'-disulfonic acid propylpropylenediamine bisulfate is 1:0.5.
preparation example 2
[0038] Preparation Example 2 of Solid Carrier Supported Catalyst: Preparation of Catalyst G2
[0039] S1: calcining carbon nanotubes at 850° C. for 13 minutes, and cooling naturally to obtain calcined carbon nanotubes;
[0040] S2: add the calcined carbon nanotubes and the active agent N,N,N',N'-tetramethyl-N,N'-disulfonic acid propylpropylenediamine bisulfate into an appropriate amount of organic solvent polyethylene glycol In alcohol (PEG-200), fully stirred at 75°C for 30 minutes, then stood still for 15 hours, filtered, and the filtered solid was fully washed with deionized water and dried completely to obtain the solid supported catalyst, named as G2;
[0041] Wherein, the mass ratio of the calcined carbon nanotubes to the active agent N,N,N',N'-tetramethyl-N,N'-disulfonic acid propylpropylenediamine bisulfate is 1:0.7.
preparation example 3
[0042] Preparation Example 3 of Solid Carrier Supported Catalyst: Preparation of Catalyst G3
[0043] S1: calcining carbon nanotubes at 900° C. for 10 minutes, and cooling naturally to obtain calcined carbon nanotubes;
[0044] S2: add the calcined carbon nanotubes and the active agent N,N,N',N'-tetramethyl-N,N'-disulfonic acid propylpropylenediamine bisulfate into an appropriate amount of organic solvent polyethylene glycol In alcohol (PEG-200), fully stirred at 80°C for 20 minutes, then stood still for 20 hours, filtered, and the filtered solid was fully washed with deionized water and dried completely to obtain the solid supported catalyst, named as G3;
[0045] Wherein, the mass ratio of the calcined carbon nanotubes to the active agent N,N,N',N'-tetramethyl-N,N'-disulfonic acid propylpropylenediamine bisulfate is 1:0.9.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com