Application of substituted benzene guanidine derivative serving as polymyxins antibiotic potentiator
A polymyxin and derivative technology, applied in the field of biomedicine, can solve problems such as environmental pollution and the impact of the application of polymyxin antibiotics, and achieve a significant effect of drug resistance reversal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Preparation of substituted benzoguanidine derivatives
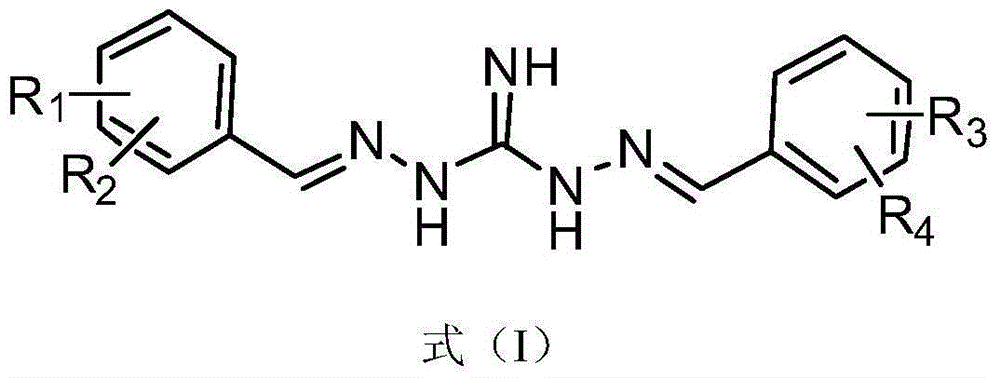
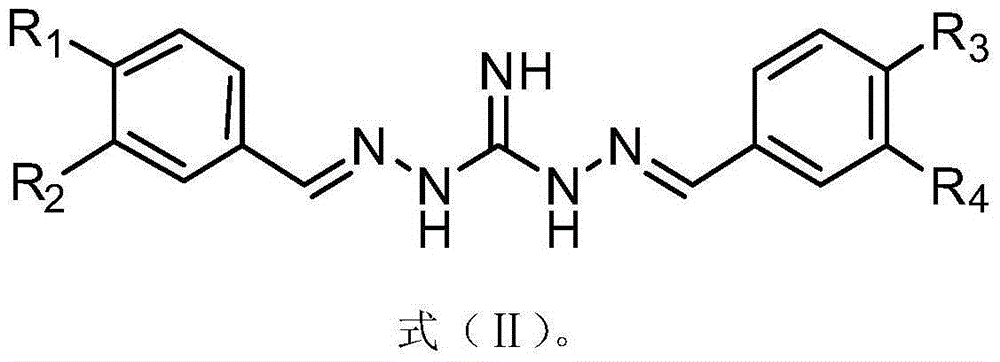
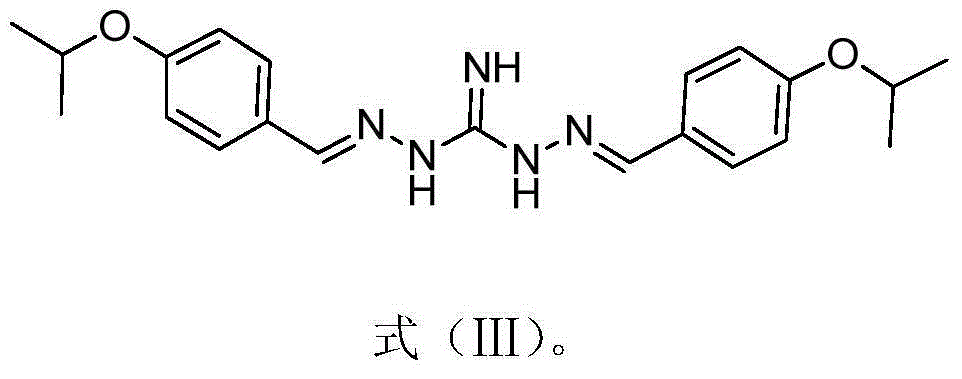
[0041] Substituted benzoguanidine derivatives, its structural formula is as shown in formula (I):
[0042]
[0043] where: R 1 , R 2 , R 3 and R 4 H, CN, NO 2 , S(=O) 2 CH 3 , C(=O)OC 1 - 8 Straight chain or branched chain alkyl, halogen, C 1 - 8 Straight chain or branched chain alkyl, C 1 - 8 Straight chain or branched haloalkyl, O-C 1 - 14 Straight chain or branched chain alkyl, O-C 3 - 8 Cycloalkyl; O-CH 2 -Ph, O-Ph, S-C 1 - 14 Straight chain or branched chain alkyl, or, S-C 1 - 14 Straight chain or branched haloalkyl;
[0044] The halogen is F, Cl, Br or I;
[0045] The haloalkyl group refers to an alkyl group substituted by F, Cl, Br or I;
[0046] The O-CH 2 The phenyl in -Ph and O-Ph refers to the phenyl that is unsubstituted or substituted by 1, 2, 3, 4 substituents, and the substituents are selected from F, Cl, Br, I, C 3 - 8 Cycloalkyl, C 1 - 8 Straight chain o...
Embodiment 2
[0055] Example 2: Synergistic effect of substituted benzoguanidine derivatives on the inhibitory activity of colistin sulfate-sensitive Escherichia coli.
[0056] The substituted benzoguanidine derivative in this example is a substituted benzoguanidine derivative hydrochloride. That is, the test was carried out with substituted benzoguanidine derivative hydrochloride.
[0057]Test the minimum inhibitory concentration (MIC) in vitro of colistin sulfate and substituted benzoguanidine derivatives to Escherichia coli (sensitive to colistin sulfate, MIC value is less than 4.0ppm) by test tube double dilution method. When 20.0ppm substituted benzoguanidine derivatives, the minimum inhibitory concentration of colistin sulfate to corresponding bacterial strains in vitro (it was to use the combined use of substituted benzoguanidine derivatives and colistin sulfate as test drug, during the test, the concentration of substituted benzoguanidine derivatives Fixed at 20.0ppm, and colistin ...
Embodiment 3
[0063] Example 3 Synergistic effect of substituted benzoguanidine derivatives on the inhibitory activity of colistin sulfate-resistant Escherichia coli.
[0064] The substituted benzoguanidine derivative in this example is a substituted benzoguanidine derivative hydrochloride. That is, the test was carried out with substituted benzoguanidine derivative hydrochloride.
[0065] Test the in vitro minimum inhibitory concentration (MIC) of colistin sulfate and substituted benzoguanidine derivatives to colistin sulfate resistant Escherichia coli (to colistin sulfate drug resistance, MIC value greater than 4.0ppm) by test tube double dilution method, At the same time, the minimum inhibitory concentration of colistin sulfate to the corresponding strains was tested in vitro when 20.0 ppm substituted benzoguanidine derivatives were respectively contained in the culture. The results showed that all test strains were resistant to colistin sulfate and all tested substituted benzoguanidine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com