Method for preparing ginkgolide K
A technology of ginkgolide and organic solvent, which is applied in the field of preparation of ginkgolide K, can solve the problems of difficult scale-up production, high reaction temperature, and unknown yield, and achieve high yield and purity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
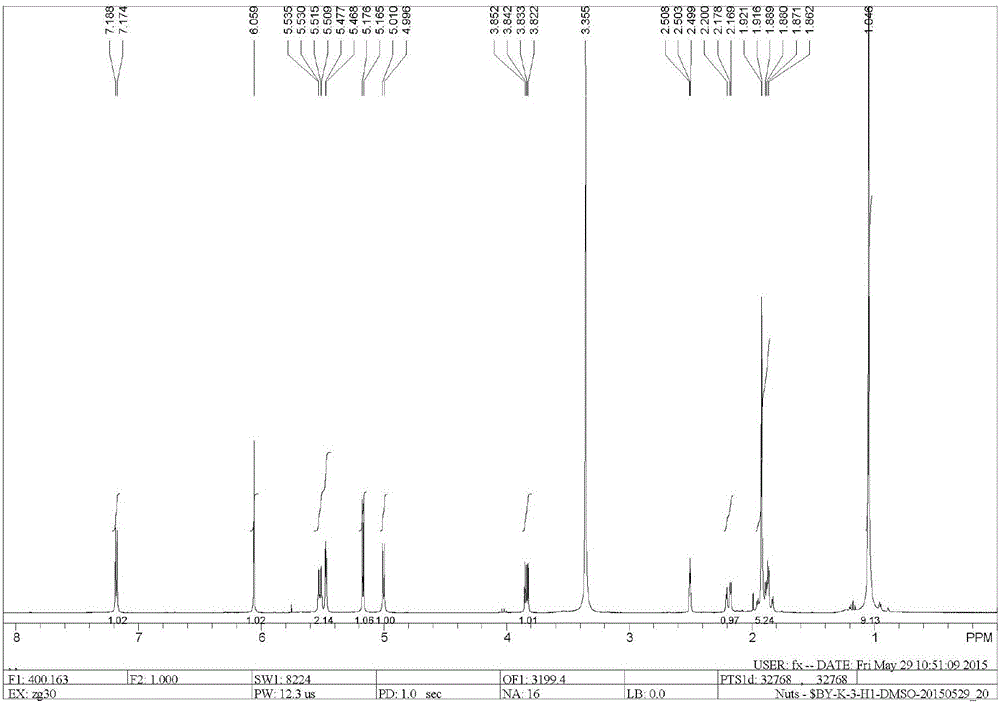
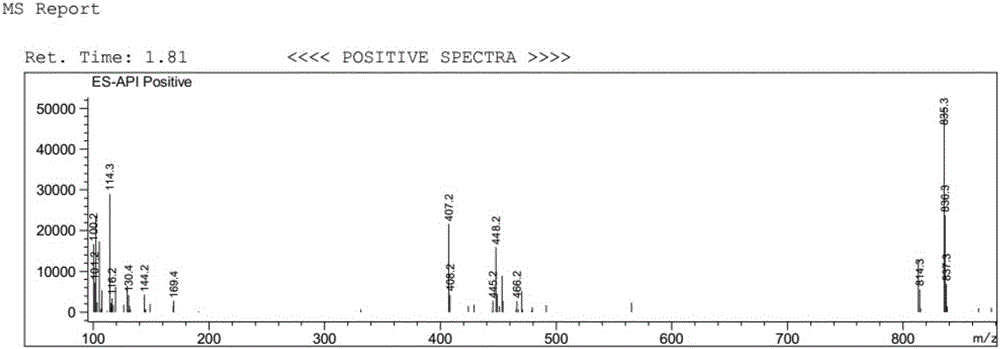
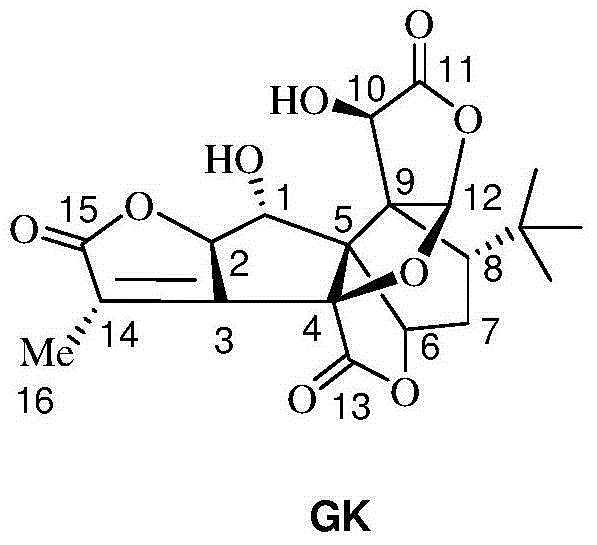
[0039] Dissolve 5 g of ginkgolide B (GB) in 100 mL of dry dichloromethane, stir and mix evenly, then cool down to -25°C under nitrogen protection. Add 10mL of DAST dropwise (that is, the volume-to-weight ratio of ginkgolide B is 2mL / g), and keep the reaction for 0.5h; then raise the temperature to room temperature, and stir the reaction until the raw material of ginkgolide B (GB) is completely reacted. Add 50 mL of purified water to the reaction solution, quench the reaction and remove the organic solution by rotary evaporation, extract the aqueous layer with ethyl acetate, wash the organic layer with saturated sodium bicarbonate solution and sodium chloride solution, and then dry it, and remove the organic solution by rotary evaporation to obtain Oil. Purification by column chromatography (petroleum ether / ethyl acetate = 2:1) yielded 1.2 g of ginkgolide K as a white solid, with a yield of 25% and an HPLC purity of 99.24%.
Embodiment 2
[0045] Dissolve 5 g of ginkgolide B in 100 mL of dry dichloromethane, stir and mix evenly, then cool down to -25°C under nitrogen protection. Add 50mL DAST dropwise (that is, the volume-to-weight ratio of ginkgolide B is 10mL / g), and keep it warm for 0.5h; Add 50 mL of purified water to the reaction solution, quench the reaction and remove the organic solution by rotary evaporation, extract the aqueous layer with ethyl acetate, wash the organic layer with saturated sodium bicarbonate solution and sodium chloride solution, and then dry it, and remove the organic solution by rotary evaporation to obtain Oil. Purification by column chromatography (petroleum ether / ethyl acetate = 2:1) yielded 1.08 g of ginkgolide K as a white solid, with a yield of 23% and an HPLC purity of 99.51%.
Embodiment 3
[0047]Dissolve 5 g of ginkgolide B in 100 mL of dry acetonitrile, stir and mix evenly, then cool down to -25°C under nitrogen protection. Add 15mL of DAST dropwise (that is, the volume-to-weight ratio of ginkgolide B is 3mL / g), and keep it warm for 0.5h; then raise the temperature to room temperature, and stir until the raw material of ginkgolide B reacts completely. Add 50mL of purified water to the reaction solution, quench the reaction, remove the organic solution by rotary evaporation, extract the aqueous layer with ethyl acetate, wash the organic layer with saturated sodium bicarbonate solution and sodium chloride solution, and dry it, then remove the organic solution by rotary evaporation Get oil. Purification by column chromatography (petroleum ether / ethyl acetate=2:1) yielded 1.3 g of ginkgolide K as a white solid, with a yield of 27% and an HPLC purity of 99.38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com