Application of pseudoalteromonas recombinant aminopeptidase
A pseudoalteromonas, aminopeptidase technology, applied in the fields of molecular biology and biochemistry, can solve the problem of less research on aminopeptidase function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
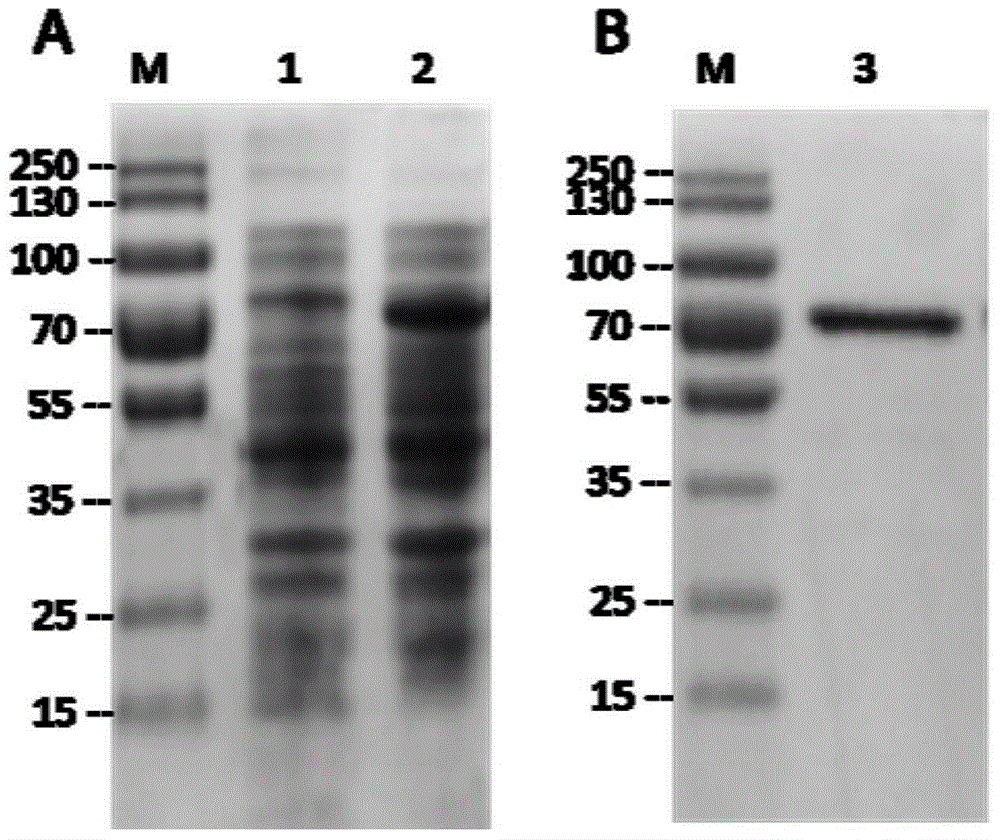
[0025] Experimental example 1: Prokaryotic recombinant expression and purification of the mature peptide coding region of P. telluritireducens 16098 aminopeptidase in vitro
[0026] 1. Construction of recombinant vector
[0027] The recombinant vector used in the present invention is the pET-30a(+) prokaryotic expression vector of Novagen company. By PCR technology, primers P1 (5'-CATATGAA CGCGATTGATCAAAACTCAT-3') and P2 (5'-CTCGAGTTATTTTATCAAATAAAGCGTC-3') with Nde I and Xho I restriction sites added at the 5' ends were used to amplify the Coding region for the mature peptide of the Pseudoalteromonas P. telluritireducens 16098 aminopeptidase from the hydrothermal vent. The reaction conditions were as follows: 94°C pre-denaturation for 5 minutes, followed by the following cycles: 94°C denaturation for 30 seconds, 65°C annealing for 30 seconds, 72°C extension for 2 minutes, a total of 35 cycles, and finally 72°C extension for 10 minutes. The amplified fragment was purified an...
experiment example 2
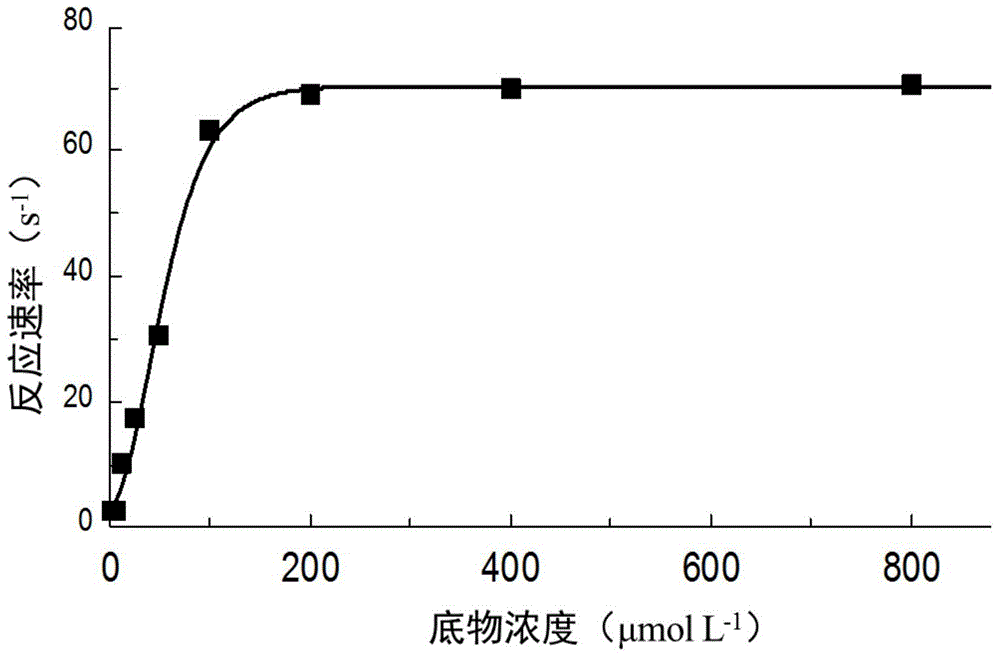
[0040] Experimental Example 2: Determination of the Km value of P. telluritireducens 16098 aminopeptidase recombinant protein Add 80 μl of buffer II to each well of a 96-well plate, 10 μl of recombinant protein (10 μg ml -1 ) and 10 μl of different concentrations of the substrate L-leucine 7-amino-4-methylcoumarin (L-leucine7-amido-4-methylcoumarin, MCA-L, Sigma), immediately in the excitation light 355nm, absorption light Continuously measure the absorbance at 440nm, calculate the enzyme reaction rate (expressed as the increase in absorbance per second dA / dt), and calculate the Km value. The control group was treated with BSA (10 μg ml -1 ) instead of recombinant aminopeptidase, each experiment was repeated three times, and the mean and standard deviation were calculated.
[0041] Experimental results: at 28°C and pH 7.2, the Michaelis-Menten equation curve of P. telluritireducens 16098 aminopeptidase recombinant protein is as follows figure 2 As shown, the estimated Km is...
experiment example 3
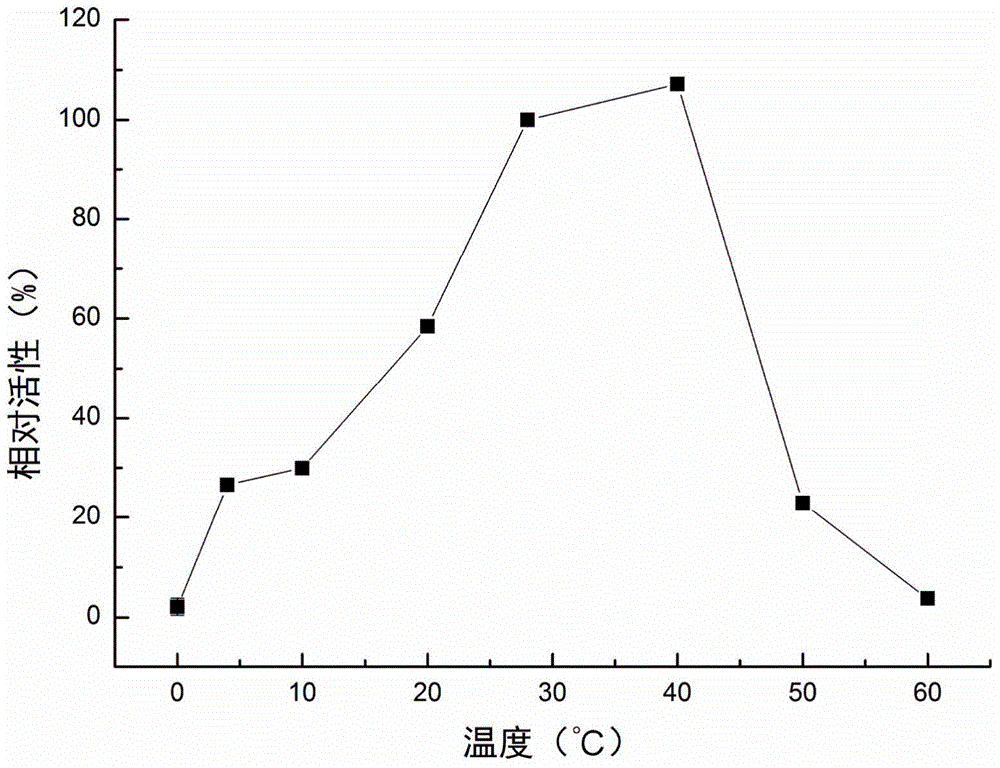
[0042] Experimental example 3: Detection of the optimal temperature of the recombinant protein of P. telluritireducens 16098 aminopeptidase
[0043] Detect the activity of recombinant protein at 0°C, 4°C, 10°C, 20°C, 28°C, 40°C, 50°C and 60°C. Add 80 μl buffer II, 10 μl recombinant protein (10 μg ml -1 ) and 10μl MCA-L (2mmol L -1 ), after reacting at the above temperature for 30 minutes, the absorbance was continuously measured under excitation light 355nm and absorption light 440nm, and the relative activity at each temperature was calculated with the enzyme activity at 28°C as 100%. Each experiment was repeated three times, and the mean and standard deviation were calculated.
[0044] Experimental results: at pH 7.2, the optimal temperature of P. telluritireducens 16098 aminopeptidase recombinant protein is 28-40°C. The relative activity at 40°C was 107.13±1.73%, slightly higher than that at 28°C but not significantly different from that at 28°C. The relative activity w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com