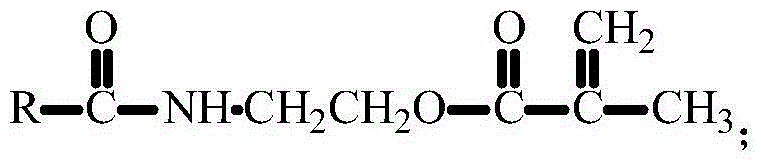Preparation method of fluorescent waterborne polyurethane acrylate
A urethane acrylate and acrylate technology is applied in the field of preparation of fluorescent water-based urethane acrylate, which can solve the problems of poor compatibility, easy migration, and difficulty in quantitatively introducing polyurethane molecular chains. The effect of improving machinability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Dissolve 3.0 g of 2-naphthylamine in 150 mL of dichloromethane, cool in an ice-water bath, add 3.41 g of isocyanoethyl methacrylate, react in an ice-water bath for 15 minutes, then react at room temperature for 16 hours, filter, and use the solid without Wash with water and ether, then recrystallize with absolute ethanol, place in a vacuum oven and dry at room temperature for 24 hours to obtain the fluorescent small molecule NUMA containing double bonds, the structural formula is:
[0037]
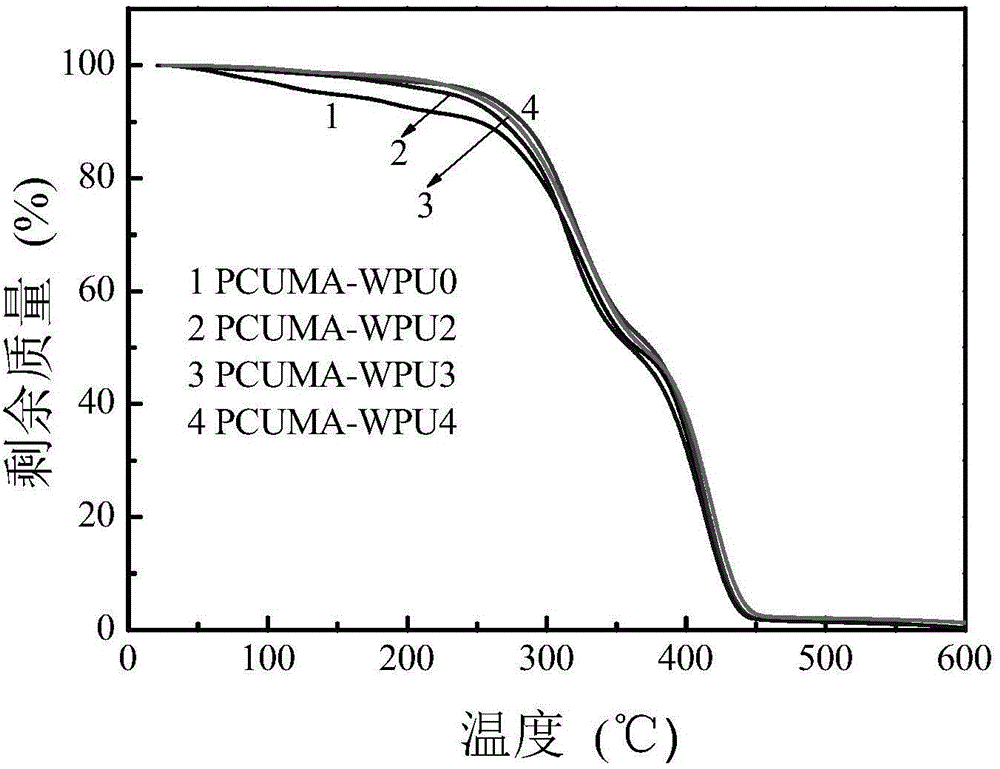
[0038] 2. Add 15.0 grams of PPG (M n=2000) into a 250mL three-necked flask, dehydrated at 110°C for 1 hour and then cooled to 50°C; 8.2 grams of TDI was added to the three-necked flask, stirred and reacted at 80°C for 2 hours, then added 1.7 grams of DMPA, 2.2 grams of DEG , 0.02 grams of DBTDL and 20 grams of butanone, stirred and reacted at a constant temperature of 70°C for 4 hours, added 0.08 grams of HQ, 1.42 grams of HEMA and stirred at a constant temperature of 70°C for...
Embodiment 2
[0040] 1. Dissolve 2.0 g of 2-aminoanthraquinone in 100 mL of dichloromethane, cool in an ice-water bath, add 1.46 g of isocyanoethyl methacrylate, react in an ice-water bath for 20 minutes, then react at room temperature for 16 hours, filter, and use the solid Wash with anhydrous ether, recrystallize with absolute ethanol, place in a vacuum oven and dry at room temperature for 24 hours to obtain a fluorescent small molecule AUMA containing a double bond. The structural formula is:
[0041]
[0042] 2. Add 20.0 grams of PCL (M n =1000) was added to a 250mL three-necked flask, dehydrated at 120°C for 1 hour and then cooled to 50°C; 14.4 grams of HDI was added to the three-necked flask, stirred and reacted at 90°C for 2 hours, and then 2.8 grams of DMBA and 4.1 grams of HDO were added , 0.02 gram of DBTDL and 30 gram of butanone, stirred and reacted at 75°C for 4 hours at a constant temperature, added 0.10 gram of BHT, 1.65 gram of HPA and stirred at a constant temperature of...
Embodiment 3
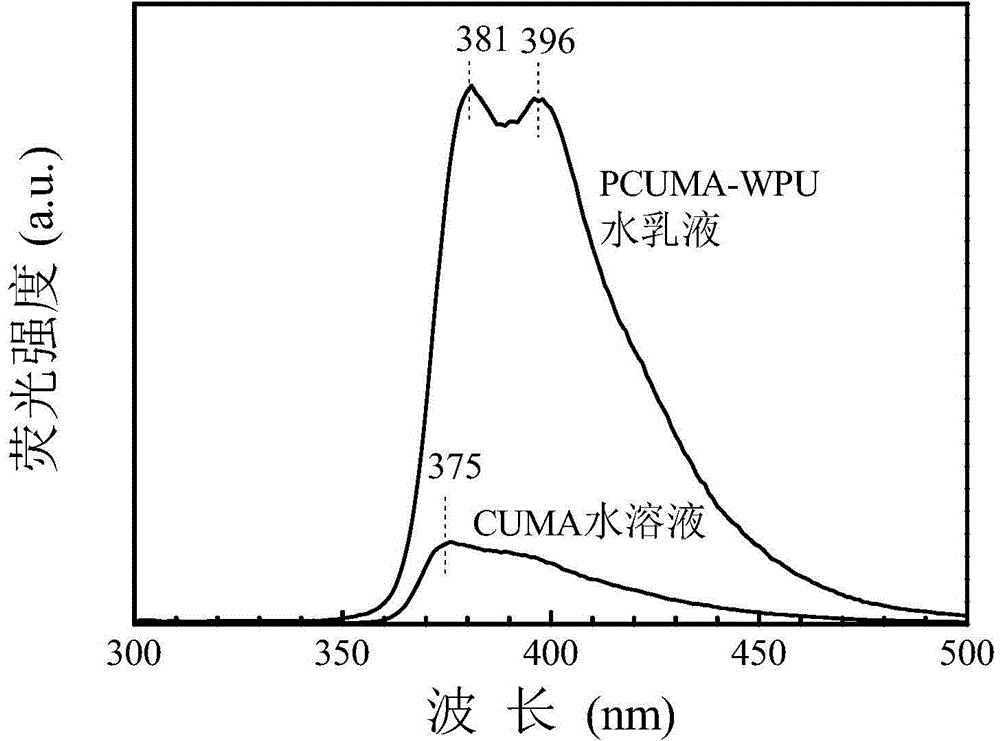
[0044] 1. Dissolve 4.0 grams of 3-amino-9-ethylcarbazole in 200 mL of dichloromethane, cool in an ice-water bath, add 3.24 grams of isocyanoethyl methacrylate, react in an ice-water bath for 15 minutes, and then react at room temperature for 16 hours , filtered, the solid was washed with anhydrous ether, and then recrystallized with absolute ethanol, placed in a vacuum oven and dried at room temperature for 24 hours to obtain a fluorescent small molecule CUMA containing a double bond. The structural formula is:
[0045]
[0046] 2. Mix 9.0 grams of PTMG (M n =2000) into a 150mL three-necked flask, dehydrated at 100°C for 0.5 hours and then cooled to 50°C; 6.0 g of IPDI was added to the three-necked flask, stirred and reacted at 90°C for 2 hours, then added 1.1 g of DMPA and 1.0 g of BDO , 0.02 grams of DBTDL and 20 grams of methyl ethyl ketone, stirred and reacted at a constant temperature of 75°C for 4 hours, added 0.05 grams of BHT, 0.93 grams of HEA and stirred at a cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com