Cefetamet pivoxil hydrochloride crystal form II and preparation method thereof
A technology of ceftazidime pivoxil hydrochloride and its crystal form, which is applied in the field of pharmaceuticals, can solve the problems of unreported compound solid-state form data, unmentioned compound crystal form research, etc., achieve good medicinal value and development prospects, and good medicinal development value, mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A crystal form II of ceftamet pivoxil hydrochloride, the preparation method of which comprises the following steps:
[0026] (1) 100 g of crude ceftazidime hydrochloride with a purity of 98.5% was fully dissolved with 300 mL of methanol at room temperature to obtain ceftazidime hydrochloride solution;
[0027] (2) Add 1500g of water to the ceftazidime pivoxil hydrochloride solution obtained in step (1), crystallize at room temperature, filter after crystallization while stirring for 5h, and air-dry at room temperature for 5h to obtain the crystal form of ceftazime pivoxil hydrochloride II 81.5g, the yield is 81.5%, and its purity is 99.6% as detected by HPLC.
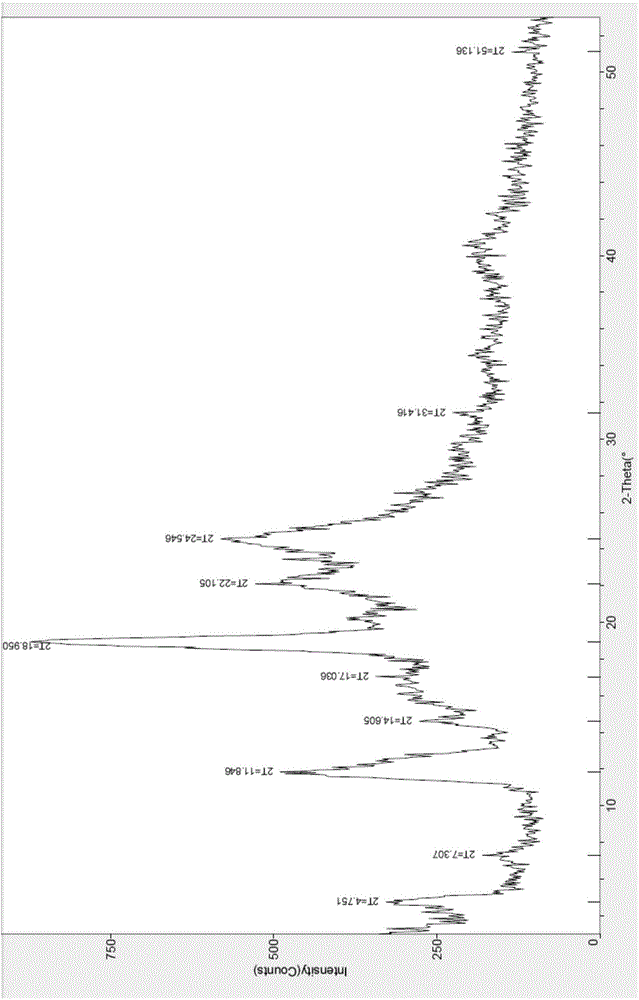
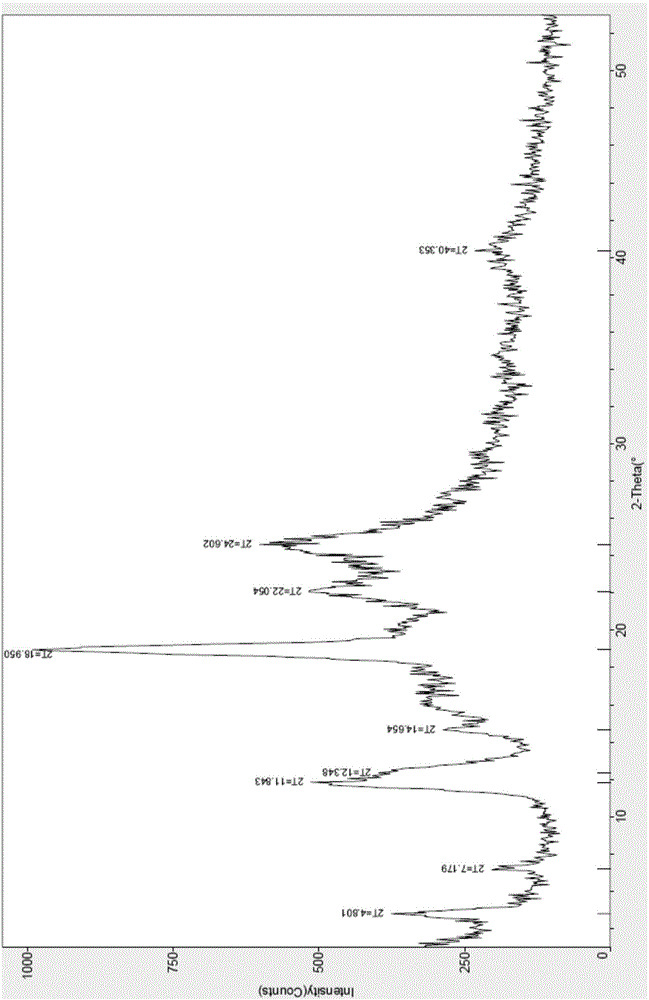
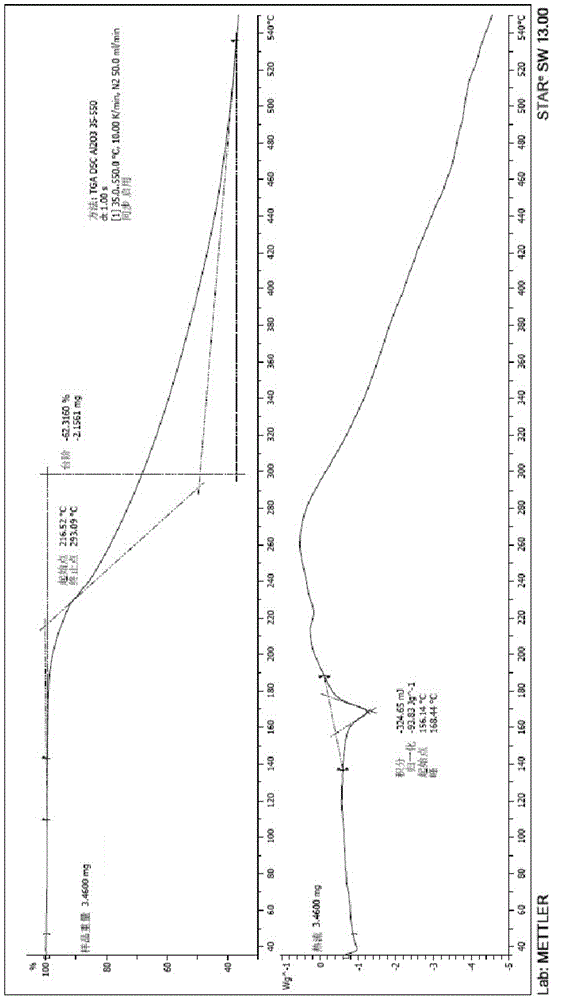
[0028] The X-ray powder diffraction pattern and DSC-TGA pattern of the crystal form II of ceftazime pivoxil hydrochloride prepared are shown in the accompanying drawings.
[0029] Stability determination: the prepared crystalline form II of ceftamet pivoxil hydrochloride was vacuum-dried at 60°C for 24 hours, an...
Embodiment 2
[0032] A crystal form II of ceftamet pivoxil hydrochloride, the preparation method of which comprises the following steps:
[0033] (1) 50 g of crude ceftazidime hydrochloride with a purity of 98.5% was fully dissolved with 100 mL of methanol at room temperature to obtain ceftazidime hydrochloride solution;
[0034] (2) add 500g water in the ceftazidime pivoxil hydrochloride solution that step (1) obtains, crystallization, room temperature crystallization, filter after the crystallization 5h while stirring, room temperature blast drying 5h, obtain ceftazime pivoxil hydrochloride The crystal form II was 43.2g, the yield was 86.4%, and its purity was 99.5% as determined by HPLC.
[0035] The X-ray powder diffraction pattern and DSC-TGA pattern of the crystal form II of ceftazime pivoxil hydrochloride prepared are shown in the accompanying drawings.
[0036] Stability determination: the prepared crystalline form II of ceftamet pivoxil hydrochloride was vacuum-dried at 60°C for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com