A kind of bismethylsiloxy tricarbonate ferrocene monomer and preparation method thereof
A technology of bismethylsiloxytricarbonate ferrocene and siloxytricarbonate ferrocene, which is applied in the field of preparation of bismethylsiloxytricarbonate ferrocene monomer and can solve the preparation conditions Harsh, limited sources of raw materials, expensive raw materials and other problems, to achieve the effect of simple preparation, low price of raw materials, wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
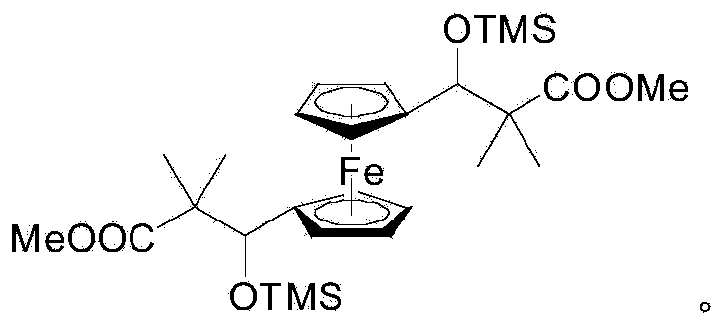
[0024] Dissolve 6.684 g of dialdehyde ferrocene (27.6 mmol) and 11.8 mL of 1-methoxy-1-(trimethylsilyloxy)-2-methyl-1-propene (55.3 mmol) in 100.0 mL of anhydrous dichloromethane ) in the dry Schlenk bottle of 250mL, under the protection of dry nitrogen, quickly drop 0.768g MgI 2 (2.8mmol) in 50.0mL ether solution, after stirring at room temperature for 6.0 hours, 10.0mL saturated NaHCO was added dropwise 3 Quenched, separated to obtain the bottom organic layer, removed the volatile solvent and dried in vacuo, further used 200 mesh to 300 mesh silica gel as the carrier, and used petroleum ether and ethyl acetate as the eluent to obtain the first fraction 5.929g 1 , 1'-bis(2-methoxycarbonyl-2-methyl-1-trimethylsilyloxy-propyl)ferrocene, the yield was 38.3%. Its chemical structure is:
[0025]
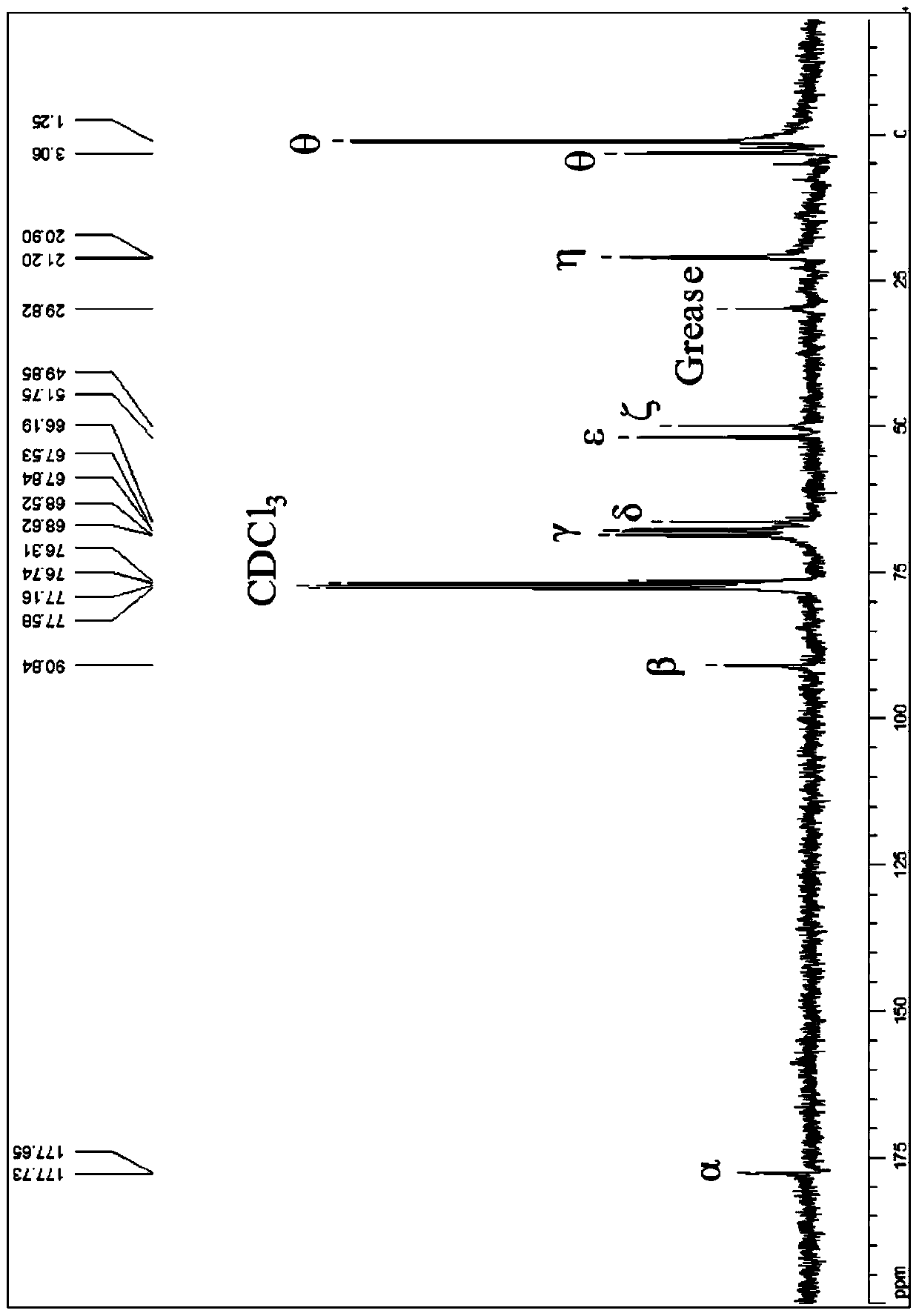
[0026] See attached figure 1 , which are respectively the compound (1,1'-bis(2-methoxycarbonyl-2-methyl-1-trimethylsilyloxy-propyl)ferrocene) prepared in this example 13 C NMR spe...
Embodiment 2
[0029]Dissolve 6.873 g of dialdehyde ferrocene (28.4 mmol) and 18.2 mL of 1-methoxy-1-(trimethylsilyloxy)-2-methyl-1-propene (85.2 mmol) in 100.0 mL of anhydrous dichloromethane ) in the dry Schlenk bottle of 250mL, under the protection of dry nitrogen, add 1.580g MgI dropwise rapidly 2 (5.7mmol) in 50.0mL ether solution, after stirring at room temperature for 6.0 hours, 10.0mL saturated NaHCO was added dropwise 3 Quenched, separated to obtain the bottom organic layer, removed the volatile solvent and dried in vacuo, further used 200-300-mesh silica gel as the carrier, and gradient elution with petroleum ether and ethyl acetate as the eluent to obtain the first fraction 7.060g 1 , 1'-bis(2-methoxycarbonyl-2-methyl-1-trimethylsilyloxy-propyl)ferrocene, the yield was 42.1%.
[0030] Compound (1,1'-bis(2-methoxycarbonyl-2-methyl-1-trimethylsilyloxy-propyl)ferrocene) prepared in this example 1 H NMR spectrum (400MHz, CDCl 3 ,ppm) and 13 C NMR spectrum (300MHz, CDCl 3 , ppm) d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com