Phosphate of epidermal growth factor receptor inhibitor, and crystal form and preparation method thereof
A phosphate and crystal form technology, which is applied in the field of chemical medicine, can solve the problems of high biotoxicity of methanesulfonic acid and unsuitability for drug synthesis, and achieve the goal of overcoming the high toxicity of methanesulfonic acid salt, meeting the requirements of bioavailability and drug efficacy, free Effect of improving alkali solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation method of phosphate:
[0058] Dissolve 10.1 mg of free base of the compound of formula (I) in 0.5 mL of acetone, add 0.1 mL of 0.2 mol / L phosphoric acid solution dropwise, and react with stirring at room temperature for 24 hours to obtain phosphate.
[0059] The phosphate product prepared by the above method, its NMR identification data are as follows:
[0060] 1H NMR(400MHz,DMSO)δ10.04(s,1H),9.03(s,1H),8.63(s,1H),8.33(d,J=5.3Hz,1H),8.27(d,J=7.9Hz ,1H),7.91(s,1H),7.53(d,J=8.1Hz,1H),7.25(dd,J=8.7,6.3Hz,2H),7.16(t,J=7.5Hz,1H),7.03 (s,1H),6.55(d,J=10.3Hz,1H),6.29(dd,J=16.9,1.9Hz,1H),5.84–5.71(m,1H),3.92(s,3H),3.88( s,3H),3.04(s,2H),2.69(s,3H),2.62(s,2H),2.41(s,6H).
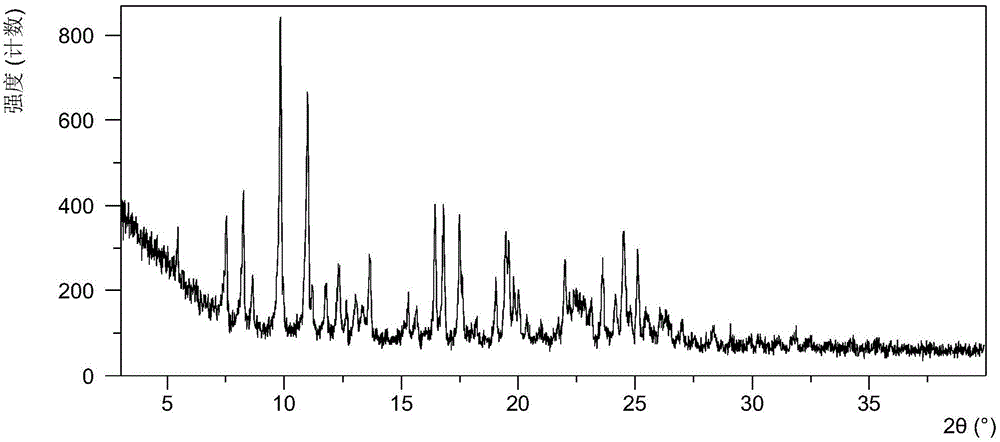
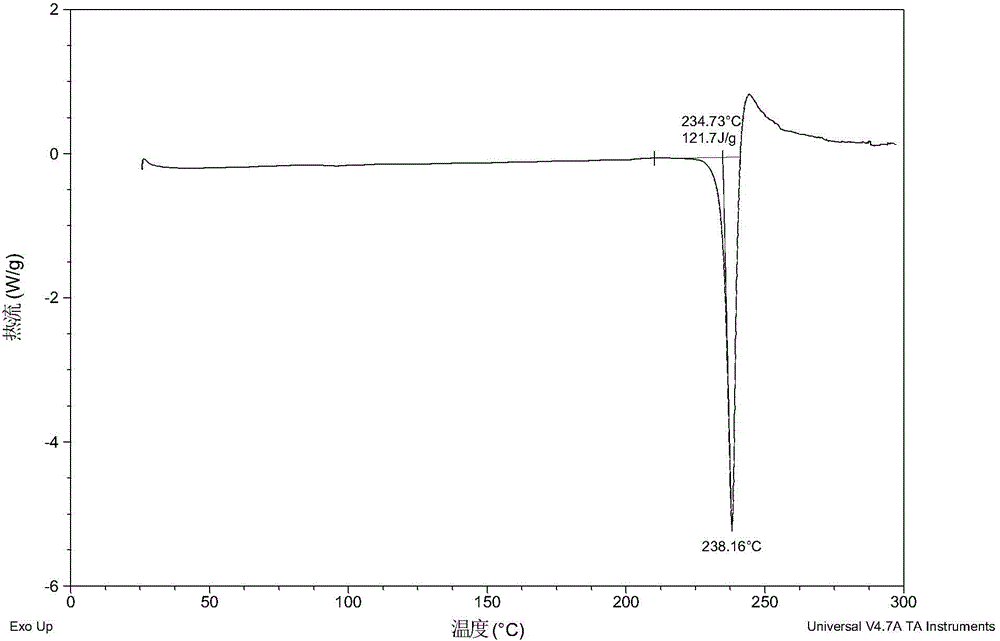
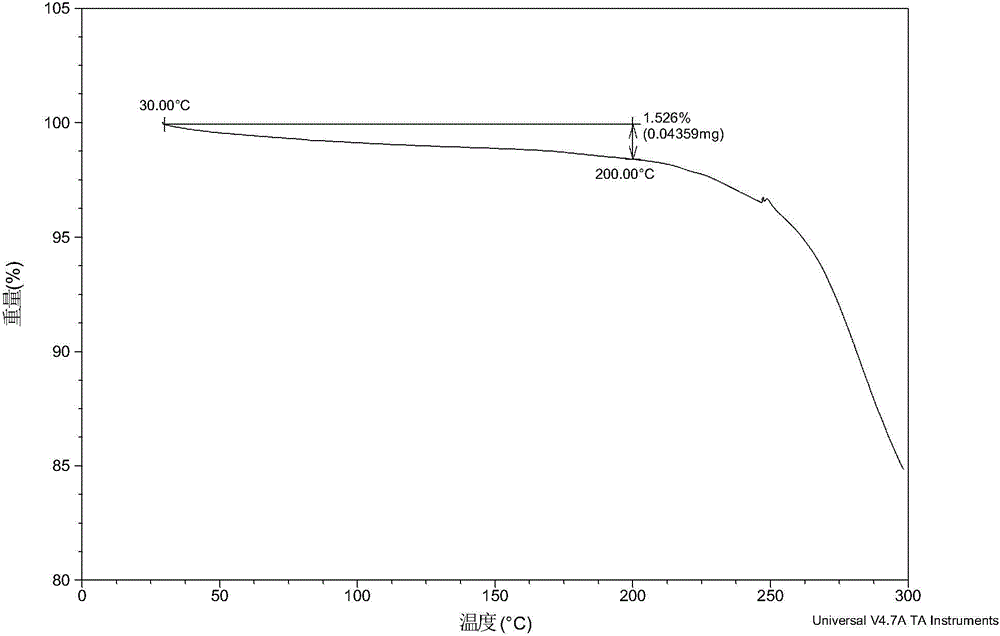
[0061] After testing, the solid obtained in this example is crystal form A, and its X-ray powder diffraction data are shown in Table 1. Its XRPD pattern is as follows figure 1 , and its DSC graph is shown in figure 2 , and its TGA figure is shown in image 3 .
[0062] Table 1 X-ray powde...
Embodiment 2
[0066] Preparation method of phosphate:
[0067] Dissolve 10.2 mg of the free base of the compound of formula (I) in 0.5 mL of ethanol, add dropwise 0.1 mL of 0.2 mol / L phosphoric acid solution, and stir at room temperature for 24 hours to obtain phosphate.
[0068] After testing, the solid obtained in this example is crystal form A, and its X-ray powder diffraction data are shown in Table 2.
[0069] Table 2 X-ray powder diffraction data of crystal form A
[0070] 2theta d interval strength% 7.43 11.91 31.68 8.17 10.83 28.63 8.57 10.32 16.45 9.76 9.06 100.00 10.70 8.27 57.18 11.85 7.47 7.92 12.25 7.22 13.48 12.85 6.89 3.59 13.60 6.51 22.65 15.20 5.83 15.23 16.37 5.41 35.12 16.78 5.28 19.18 17.49 5.07 17.43 19.51 4.55 41.64 21.51 4.13 24.69 22.56 3.94 20.93 23.55 3.78 13.26 24.47 3.64 37.67
[0071] 25.06 3.55 31.75 25.64 3.47 16.14 ...
Embodiment 3
[0073] Comparative study on dynamic solubility of compound phosphate and free base of formula (I):
[0074] The formula (I) compound phosphate and free base were respectively treated with H 2 O and SGF (simulated gastric fluid, artificial gastric juice) at pH 1.8 were prepared into a saturated solution, and the content was measured by high performance liquid chromatography (HPLC) after 1 hour, 4 hours and 24 hours respectively. The experimental results are shown in Table 3.
[0075] table 3
[0076]
[0077] The results showed that the solubility of phosphate in water was much higher than that of free base in water, and during the analysis, it was found that free base was degraded in SGF within 24 hours, and its stability in biological media was poor, which was not conducive to drug production. The invention overcomes the problems of low solubility of free base and instability in biological media by forming phosphate, and achieves the effect of improving bioavailability. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com