Methods, compositions and kits for treating, modulating, or preventing ocular angiogenesis or fibrosis in a subject using a galectin protein inhibitor
A technology of galectin and angiogenesis, applied in the field of using galectin protein inhibitors for treating, regulating or preventing ocular angiogenesis or fibrosis of subjects, compositions and kits, capable of solving Surgical recurrence and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
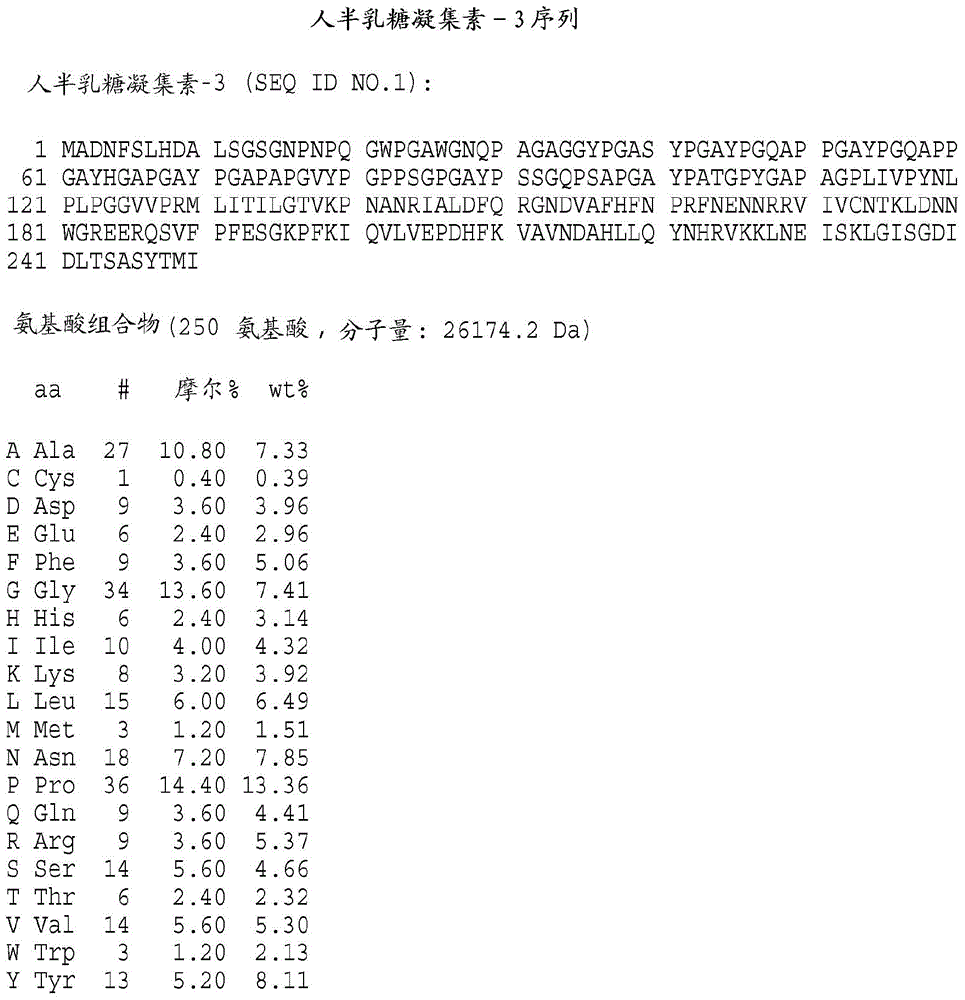
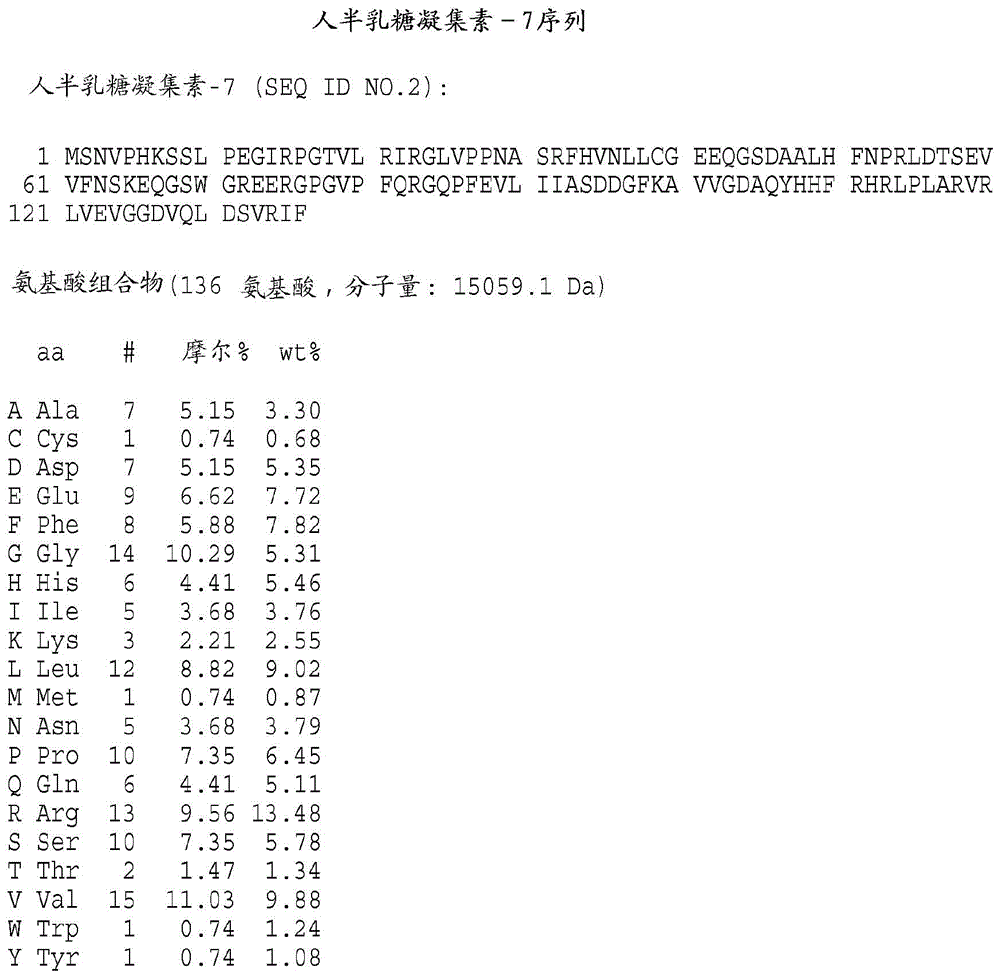
[0343] Preparation of galectin protein
[0344] It will be understood by those of ordinary skill in the art that the galectins of the present invention may be obtained from any available source. These include, but are not limited to, proteins isolated from natural sources, produced recombinantly or synthetically, for example, by solid phase procedures. According to the present invention, polynucleotide sequences, which encode galectin-3, galectin-7 or galectin-8, can be directed to the galectin of the present invention in a suitable host cell Recombinant DNA molecules for the expression of kinetochores are used. Cherayil et al., supra; Madsen et al., supra; and Hadri et al., supra; detailed galectin-7 and galectin-8 descriptions, respectively, of human galectin-1, human galectin- 3 clones. For the expression of biologically active human galectin-1, galectin-3, galectin-7 or galectin-8 encoded by the nucleotide sequence 3. Galectin-7, Galectin-8 or their functional equiva...
Embodiment 1
[0371] Example 1. For the synthesis of bis(3-deoxy-3-(3-fluorophenyl-1H-1,2,3-triazol-1-yl) - Materials and equipment for β-D-galactopyranosyl)sulfane
[0372] Bis(3-deoxy-3-(3-fluorophenyl-1H-1,2,3-triazol-1-yl)-β-D-galactopyranosyl)sulfane (TD139) was produced by Professional Post System (Profs) provided. Hakon Leffler and Ulf Nilsson (Lund University, Sweden), and prepared by administering the materials and methods of the invention described herein.
[0373] Melting points were recorded on a Kofler apparatus (Reichert) and were uncorrected. Proton NMR (1H) spectra were recorded using a Bruker DRX400 (400 MHz) or a Bruker ARX300 (300 MHz) spectrometer; the multiplicity was represented as singlet (s), peak (d), doublet (dd), triplet (t ), apparent triplet (ATD) or doublet apparent triplet (ATD) citations. Carbon NMR (13C) spectra were recorded using a Bruker DRX400 (100.6 MHz) spectrometer. Spectra were assigned using COZY, HMQC and DEPT experiments. All chemical shi...
Embodiment 2
[0378] Example 2. Phenyl 2-O-acetyl-4,6-O-benzylidene-1-thio-3-O-trifluoromethanesulfonyl- Synthesis of β-D-galactopyranoside (structural formula 2 in scheme 1)
[0379] Compound 1 (10.5 g, 29.2 mmol) was dissolved in dry pyridine (4.73 mL, 58.4 mmol) and dry CH 2 Cl 2 (132 ml). The reaction mixture was cooled with stirring to -20 °C (ice and NaCl bath, ratio 3:1). in N 2 Atmospheric conditions, slowly increase Tf 2 O (5.68 mL, 33.6 mmol) was added. The reaction mixture was monitored by TLC (heptane:EtOAc 1:1 and toluene:acetone 10:1). When the reaction was complete, AcCl (2.29 mL, 32.1 mmol) was added and stirred while the temperature was allowed to rise to room temperature. This mixture was monitored by TLC (heptane:EtOAc 1:1; and toluene:acetone 10:1). When the reaction is complete, use CH 2 Cl 2 Quenched with 5% HCl, NaHCO 3 (saturated) and NaCl (saturated) for washing. Among them, the organic layer uses MgSO 4 It was dried, filtered and concentrated under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com