Family of aryl, heteroaryl, o-aryl and o-heteroaryl carbasugars
An aryl and group technology, applied in the field of treatment or prevention of diabetes and obesity, can solve problems that have not yet been enumerated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
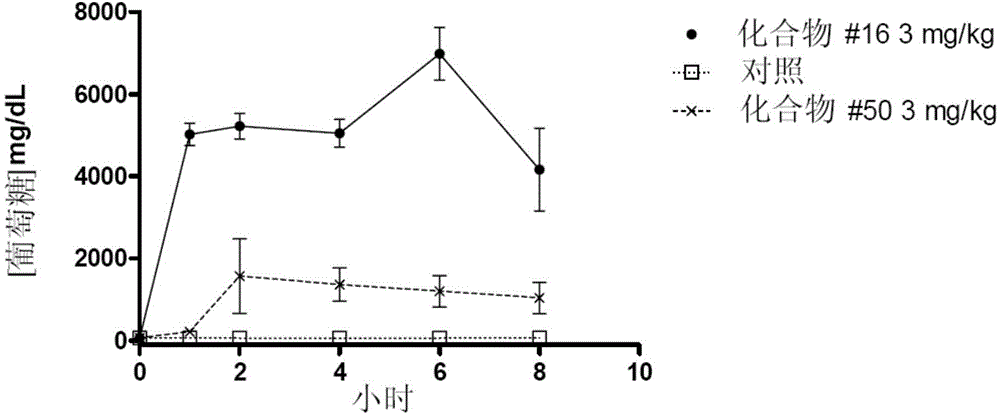
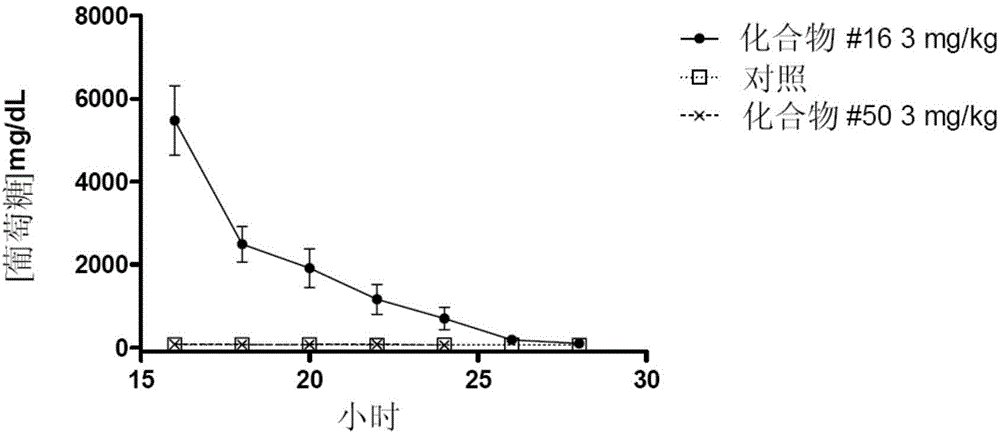
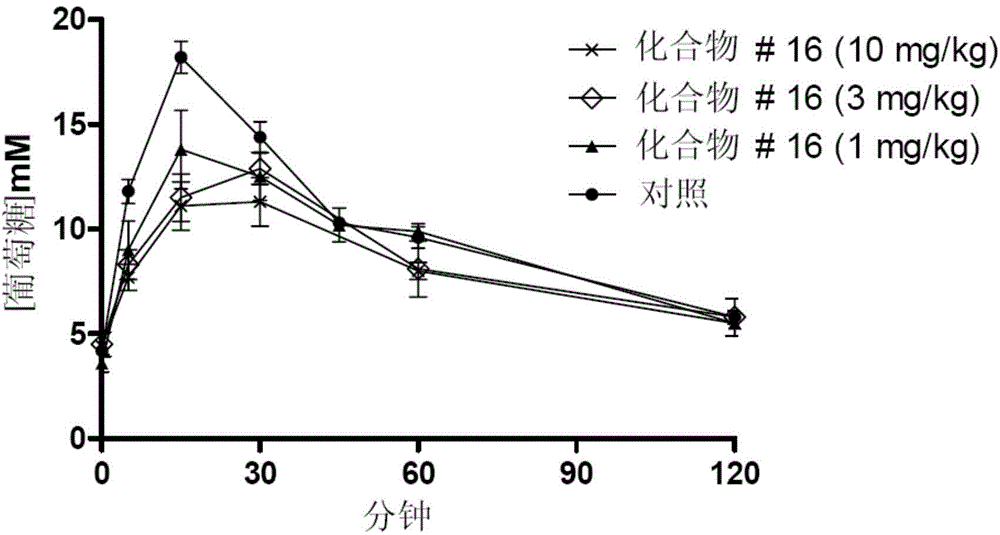
Image
Examples
Embodiment
[0330] 1. Preparation of the compound of the present invention
[0331] Abbreviations encountered in the text are defined as follows:
[0332] Acetyl
[0333] ADDP Dipiperidine azodicarboxylate
[0334] Bn benzyl
[0335] cat. Catalytic
[0336] DAST diethylaminosulfur trifluoride
[0337] DCM dichloromethane
[0338] de diastereomeric excess
[0339] DMAP 4-Dimethylaminopyridine
[0340] DMF Dimethylformamide
[0341] DMSO Dimethyl Sulfoxide
[0342] eq. Equivalent
[0343] ESI electrospray ionization
[0344] g grams
[0345] Hz Hertz
[0346] mg mg
[0347] MHz megahertz
[0348] min. minute
[0349] mL milliliter
[0350] mmol millimole
[0351] mM millimole / liter
[0352] μmol micromole
[0353] nmol Nanomole
[0354] NMR nuclear magnetic resonance
[0355] oral
[0356] PEG polyethylene glycol
[0357] QS appropriate amount
[0358] Rf blocker
[0359] rt room temperature
[0360] TFAA Trifluoroacetic anhydride
[0361] THF Tetrahydrofuran
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com