Preparation method of deuterated pimozide
A pimozide and deuterated technology, applied in the field of preparation of deuterated pimozide, can solve problems such as undiscovered reports, and achieve the effects of easy purification, high yield and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
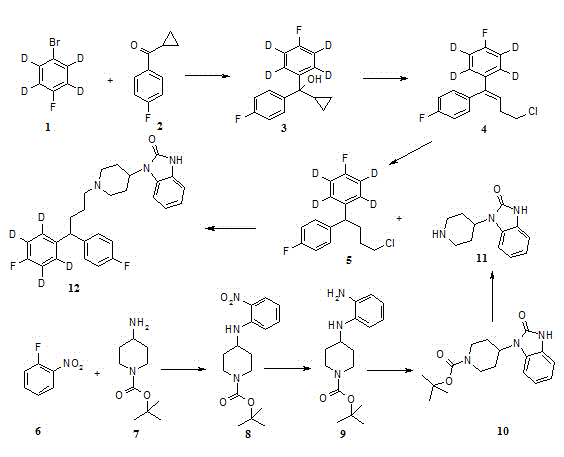
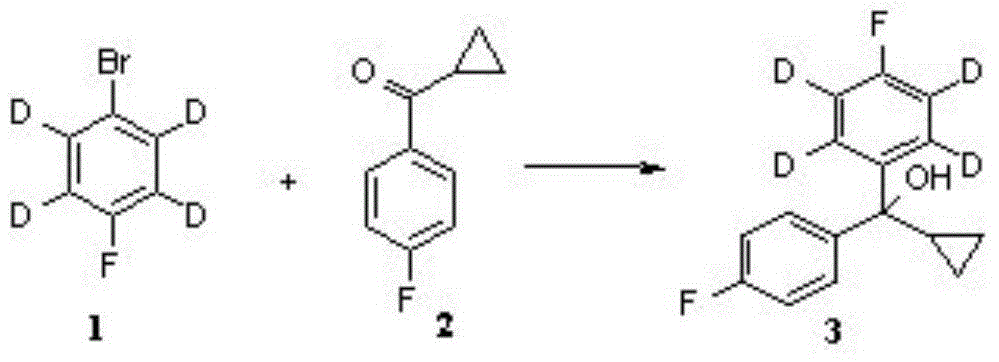
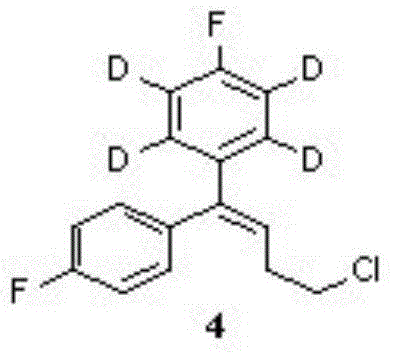
[0029] Such as figure 1 For the preparation process of deuterated pimozide picture .
[0030] The preparation of intermediate 3: get the magnesium powder of 0.43 gram and suspend in 3ml tetrahydrofuran, add the iodine of catalyst amount, the mixture is warmed up to 60 ℃, dropwise the p-fluorobromobenzene (being compound 1) of 3 grams deuterated in 7ml tetrahydrofuran The solution was added dropwise for about 10min. After the dropwise addition, the reaction was heated to 70°C for 1.5 hours to obtain the Grignard reagent of deuterated p-fluorobromobenzene, and then the obtained Grignard reagent was cooled to room temperature, and 2.75 grams of intermediate 2 were added thereto. The tetrahydrofuran solution was reacted at 40°C for 1 hour, then the reaction solution was lowered to room temperature, saturated ammonium chloride aqueous solution was added, extracted with acetic acid, the organic phase was washed with saturated sodium chloride, the organic phase was dried, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com