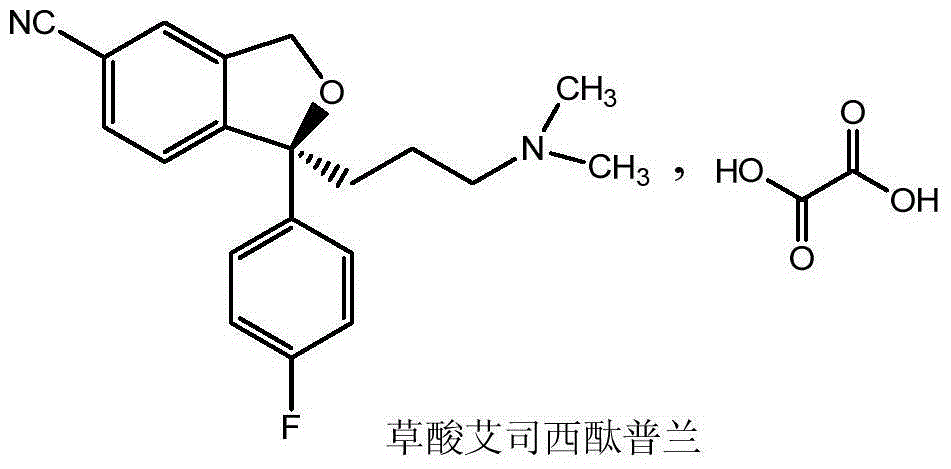
Escitalopram oxalate related substance and preparation method thereof
A technology of escitalopram oxalate and related substances, which is applied in the field of escitalopram oxalate related substances and its preparation, and can solve the problems of difficult degradation, separation, limited structural stability of the reaction degree, no compound synthesis reports, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
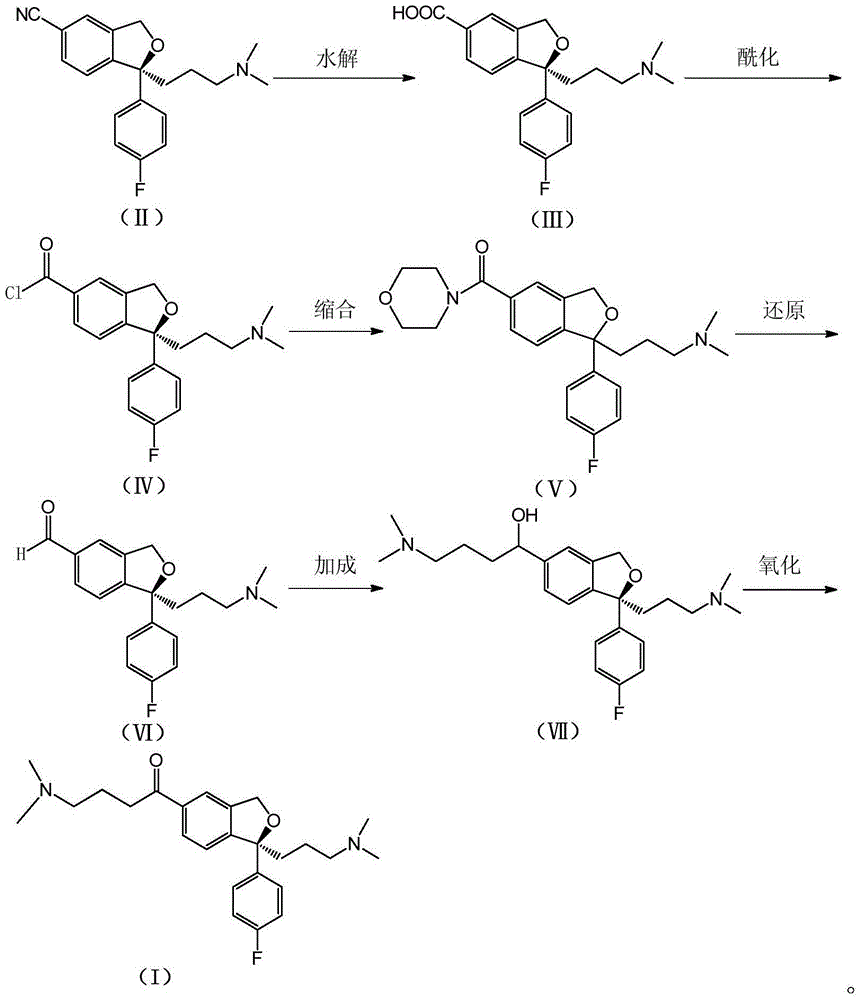
[0030] Put compound (II) (50.0g) into a reaction flask, add water (800ml) and NaOH (25.0g), stir and heat, and reflux for 24h. Cool the reaction solution to room temperature, extract with ethyl acetate (800ml×2), adjust the pH of the aqueous layer to about 5 with 2M hydrochloric acid, then concentrate under reduced pressure to remove water, add acetone (800ml) to the residue and stir for 30min, filter, and the filtrate Concentrate and treat once with acetone (800ml). After the filtrate was concentrated, Compound (III) (48.4 g) was obtained as a white solid with a yield of 91.4%.
Embodiment 2
[0032] Put compound (III) (48.4g) into a 1L reaction flask, add thionyl chloride (500ml), stir and heat under reflux for 2 hours. Then concentrated under reduced pressure to remove the solvent, and carried twice with toluene to obtain compound (IV) as a light brown oil, which was directly used in the next step.
Embodiment 3
[0034] Put the compound (IV) obtained in the previous step into THF (600ml), stir and cool down. The temperature was controlled at 0°C, and a mixture of morpholine (16.3g) / triethylamine (38.6g) in THF (200ml) was added dropwise. After dropping, the temperature was raised naturally for 1 hour. The solvent was then concentrated under reduced pressure, and the residue was added to water (600ml) and extracted with chloroform (500ml x 2). The organic layers were combined, washed with water and brine successively, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain compound (V) (42.9 g) as a brown-yellow oil, with a two-step yield of 73.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com