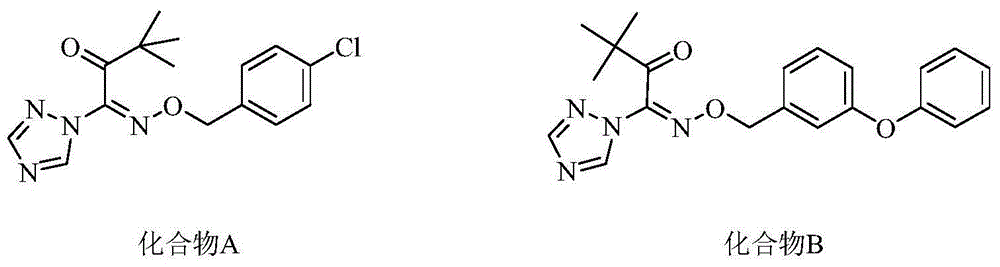
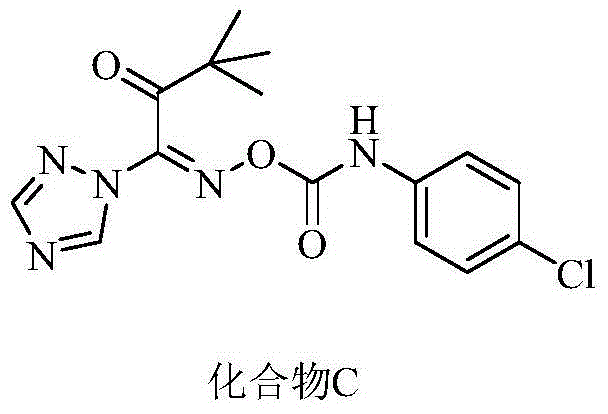
Oxime-containing carboxylate compound and use thereof
A technology for ester compounds and carboxylic acids, applied in the fields of chemicals, applications, organic chemistry for biological control, and can solve problems such as herbicide resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Example 1 Preparation of Compound 16
[0171]
[0172] 1) Preparation of intermediate (IV-1)
[0173] Add 69g (1mol) of triazole to a 1000mL three-neck bottle filled with 300mL of DMF, add 165.6g (1.2mol) of potassium carbonate, slowly add 134.5g (1mol) of chloropinacolone dropwise, stir at room temperature, and slowly Raise the temperature to 80°C and react for 4 hours. After the reaction is monitored by liquid chromatography, add 500mL of water, extract with 150mL×3 ethyl acetate, and dry the organic layer to obtain 120g of intermediate (IV-1). The product is a yellow solid . It was directly used in the next step without purification.
[0174] 2) Preparation of intermediate (III-1)
[0175] Weigh 0.83g (0.036mol) of sodium metal, cut it into sodium wire and put it into a reaction bottle filled with 50mL of ethanol in batches. After the sodium wire is completely reacted, raise the temperature to 80°C and reflux until no bubbles are released. Put the reaction sol...
Embodiment 2
[0179] Example 2 Preparation of Compound 23
[0180]
[0181] 1) Slowly add 3.6g (0.01mol) of acid (II-2) into a two-necked bottle containing 100ml of thionyl chloride, add 2 drops of DMF, heat to reflux until the solution is clear, continue heating for 1h, and evaporate under reduced pressure Excess thionyl chloride yielded 3.8 g of intermediate (II-2) as a colorless oil.
[0182] 2) According to the method described in step 4) of Example 1, 0.6 g of compound was prepared by reacting 1.0 g (0.0028 mol) of intermediate (II-2) with 0.5 g (0.0028 mol) of intermediate (III-1) (23), yellow oil. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )δppm1.30(9H,s), 7.11-7.17(2H,m), 7.33(2H,d), 7.67(1H,d), 7.84(1H,s), 8.25(1H,d), 8.91(1H ,s).
Embodiment 3
[0183] Example 3 Preparation of Compound 26
[0184]
[0185] 1) According to the method described in step 1) of Example 2, the acid of (II-2) was replaced by the acid of (II-3) to obtain the intermediate (II-3) as a colorless oil.
[0186] 2) According to the method described in step 4) of Example 1, 1.0 g (0.005 mol) of intermediate (II-3) was reacted with 1.0 g (0.005 mol) of intermediate (III-1) to obtain 1.3 g of compound (26), white solid, melting point 141.0°C. 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 ) δppm 1.42 (9H, s), 2.29 (3H, s), 4.14 (3H, s), 8.09 (1H, s), 9.32 (1H, s).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com