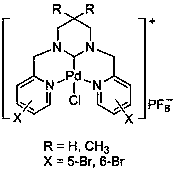
Ring enlargement azoheterocyclic carbene-palladium compound containing picolyl
A technology of heterocyclic carbene and pyridine methyl is applied to pincer-shaped ring-expanded nitrogen heterocyclic carbene palladium compound and its application field in catalyzing C-N coupling reaction, and achieves high-efficiency catalytic performance, high catalytic yield and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
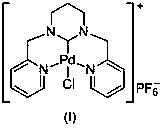
[0031] In a 100mL round-bottomed flask, mix pyridine-2-carbaldehyde (30mmol, 3.21g) and 1,3-propylenediamine (15mmol, 1.11g) in 30mL of methanol, react at room temperature for 5.0 hours, and slowly Add NaBH 4 (120mmol, 4.54g), the temperature was slowly raised to 70°C for 5 hours, cooled to room temperature, and the reaction solution was spin-dried. Then use 50mL CH 2 Cl 2 Dissolved, filtered, and the filtrate was spin-dried to obtain a yellow oily sticky substance. After dissolving the above-mentioned yellow oily viscous substance in 30 mL of methanol, an equimolar amount of formaldehyde aqueous solution was added thereto, and after stirring at room temperature for 3 hours, the solvent was removed under reduced pressure, separated and purified by column chromatography to obtain 1,3-bis(2-pyridylmethyl) Hexahydropyrimidine (3.50 g, 87% yield).
[0032] In a 100mL round bottom flask, 1,3-bis(2-pyridylmethyl)hexahydropyrimidine (5mmol, 1.34g) was dissolved in 30mL of ethylen...
Embodiment 2
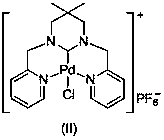
[0035] Mix pyridine-2-carbaldehyde (30mmol, 3.21g) and 2,2-dimethyl-1,3-propanediamine (15mmol, 1.53g) in 30mL methanol, react at room temperature for 7.0 hours, and stir in an ice-water bath Add NaBH slowly 4 (120mmol, 4.54g), slowly warming up to 70°C for 5 hours. The reaction was stopped, cooled to room temperature, and the reaction solution was spin-dried. Then use 50mL CH 2 Cl 2 Dissolved, filtered, and the filtrate was spin-dried to obtain a yellow oily viscous substance. After dissolving the above-mentioned yellow oily viscous substance in 30 mL of methanol, an equimolar amount of formaldehyde aqueous solution was added thereto, and after stirring at room temperature for 3 hours, the solvent was removed under reduced pressure, separated and purified by column chromatography to obtain 3,3-dimethyl-1,3 - Bis(2-pyridylmethyl)hexahydropyrimidine (3.96 g, 89% yield).
[0036] Dissolve 3,3-dimethyl-1,3-bis(2-pyridylmethyl)hexahydropyrimidine (5mmol, 1.48g) in 30mLDME, th...
Embodiment 3
[0039] Mix 5-bromopyridine-2-carbaldehyde (30mmol, 5.58g) and 1,3-propanediamine (15mmol, 1.11g) in 30mL of methanol, react at room temperature for 5.0 hours, slowly add NaBH under ice-water bath stirring 4 (120mmol, 4.54g), slowly warming up to 70°C for 5 hours. The reaction was stopped, cooled to room temperature, and the reaction solution was spin-dried. Then use 50mL CH 2 Cl 2 Dissolved, filtered, and the filtrate was spin-dried to obtain a yellow oily viscous substance. After dissolving the above-mentioned yellow oily viscous substance in 30 mL of methanol, an equimolar amount of formaldehyde aqueous solution was added thereto, and after stirring at room temperature for 3 hours, the solvent was removed under reduced pressure, separated and purified by column chromatography to obtain 1,3-bis(5-bromo-2 -Pyridylmethyl)hexahydropyrimidine (5.30 g, 83% yield).
[0040] 1,3-bis(5-bromo-2-pyridylmethyl)hexahydropyrimidine (5mmol, 2.13g) was dissolved in 30mL DME, and NBS (5m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com