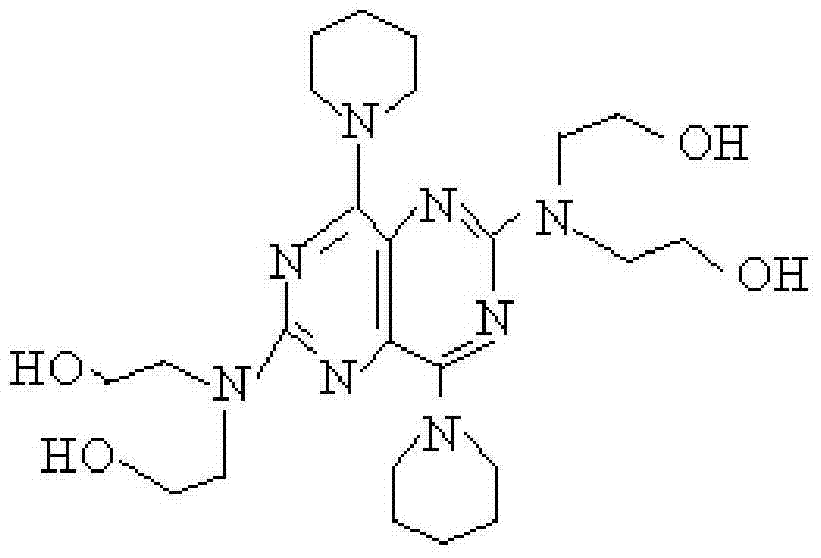
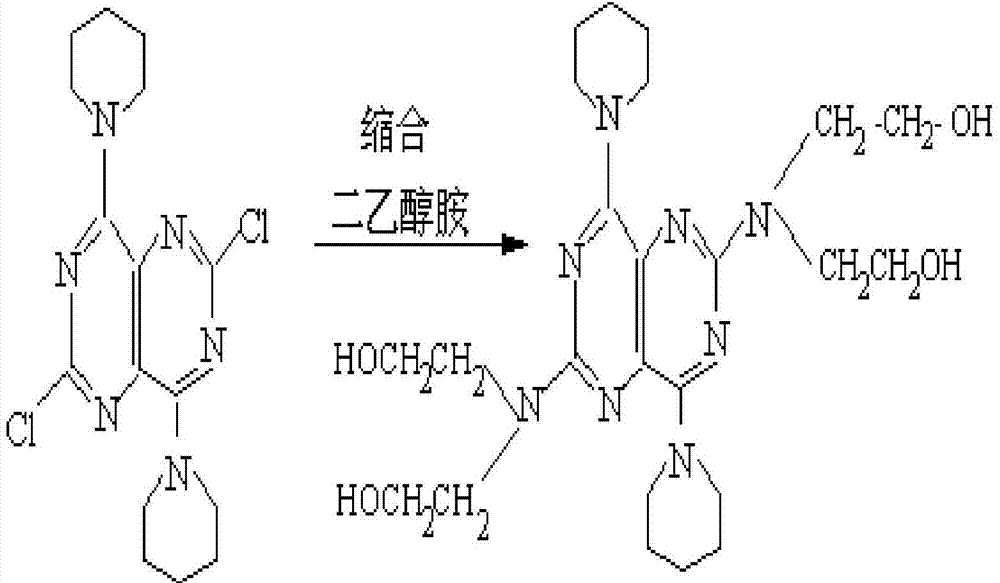
Purification technology of dipyridamole
A technology of dipyridamole and purification method, which is applied in the field of pharmaceutical preparation and can solve the problems of difficulty in separating dipyridamole and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Put the solvent methanol and the dipyridamole crude product with an original purity of only 90% into the reaction pot at a mass ratio of 3.6:1, heat it in a water bath to 50°C, keep stirring and mix for 0.5 hours, and then add p-toluenesulfonate Acid and 1-(3-aminophenyl)-3-methyl-2-imidazolinone, the mass ratio of p-toluenesulfonic acid and dipyridamole crude product is 1:1, 1-(3-aminophenyl The mass ratio of )-3-methyl-2-imidazolinone to p-toluenesulfonic acid is 0.2:1; stir to dissolve it completely; cool the solution to 6°C, keep it warm for 2.5 hours, centrifuge the material, and dry it , washed with methanol, centrifuged again to shake off the material, and dried to obtain the sulfonate intermediate product;
[0018] (2) Mix the sulfonate intermediate product obtained in step (1) with 70% (solute mass fraction) methanol solution at a mass ratio of 1:6, heat and dissolve at 50°C to form a homogeneous solution, and heat at 50°C Insulate for 0.5 hours, add liqui...
Embodiment 2
[0021] (1) Put the solvent methanol and the dipyridamole crude product with an original purity of only 87.5% into the reaction pot at a mass ratio of 3.6:1, heat it in a water bath to 50° C., keep stirring and mix for 0.5 hours, and then add p-toluenesulfonate Acid and 1-(3-aminophenyl)-3-methyl-2-imidazolinone, the mass ratio of p-toluenesulfonic acid and dipyridamole crude product is 1:1, 1-(3-aminophenyl The mass ratio of )-3-methyl-2-imidazolidinone to p-toluenesulfonic acid is 0.2:1; stir to dissolve it completely; cool the solution to 8°C, keep it warm for 3 hours, centrifuge the material, and dry it , washed with methanol, centrifuged again to shake off the material, and dried to obtain the sulfonate intermediate product;
[0022] (2) Mix the sulfonate intermediate product obtained in step (1) with 70% (solute mass fraction) methanol solution at a mass ratio of 1:5, heat and dissolve at 50°C to form a homogeneous solution, and Insulate for 0.5 hours, add liquid alkali ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com