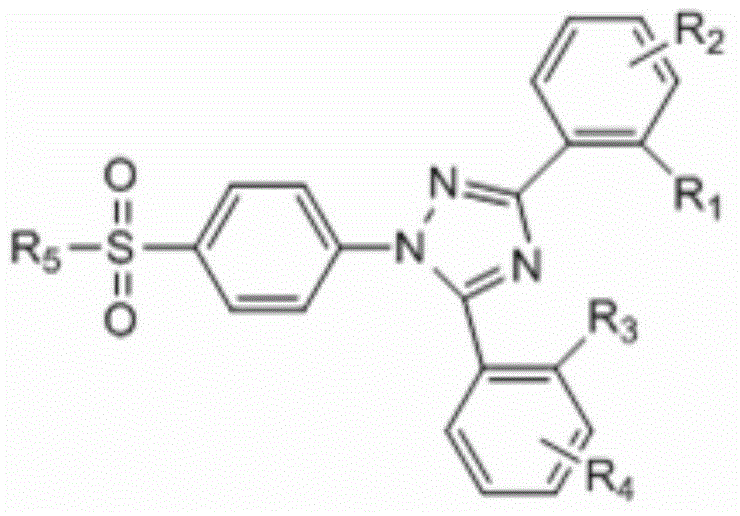
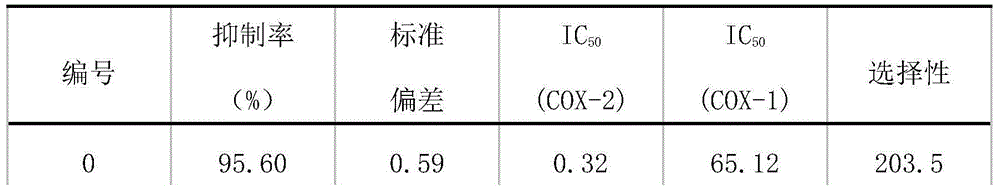
Epoxidase-2 selective inhibitor as well as preparation method and application thereof
A cyclooxygenase and selective technology, applied in the field of medicine, can solve the problems of inappropriate COX-2/COX-1 selectivity, side effects, and limited COX-2 inhibitors, and achieve cheap and easy-to-obtain raw materials and synthetic routes Effects of Short, Significant Selective Inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 (compound 1)
[0035] The preparation method of 4-[3,5-bis(2-hydroxyphenyl)-1-(1,2,4-triazolyl)]benzenesulfonamide, the steps comprising:
[0036] (1) Add 40ml of xylene and salicylic acid (17.3g, 120mmol) into a 250ml reaction bottle, slowly add 10ml of thionyl chloride with a dropping funnel, leave it for 30min after the dropwise addition, add salicylamide (11.2g, 80mmol), stirred and reacted at room temperature (20-25°C) for 5h, tracked the reaction by TLC, after the reaction was complete, evaporated the organic solvent under reduced pressure to obtain a yellow viscous solid, added 50ml of ethanol, heated to reflux for 1h, and cooled to room temperature , stood still, and 7.6g of yellow powdery solid compound 1a was precipitated.
[0037] (2) Add compound 1a (0.72g, 3mmol) and p-hydrazinobenzenesulfonamide (0.67g, 3.5mmol) to 25ml of methanol, heat to reflux for 10h, follow the reaction by thin-layer chromatography, and cool to room temperature after the r...
Embodiment 2
[0039] Example 2 (compound 1)
[0040] The preparation method of 4-[3,5-bis(2-hydroxyphenyl)-1-(1,2,4-triazolyl)]benzenesulfonamide, the steps comprising:
[0041] (1) Add salicylamide (11.2g, 80mmol) and 40ml of xylene into a 250ml reaction bottle, heat to dissolve, slowly drop salicyloyl chloride (22.7g, 0.12mol) into the reaction solution, after adding , stirred at room temperature for 5 hours, followed by thin-layer chromatography. After the reaction was complete, the organic solvent was evaporated under reduced pressure to obtain a yellow viscous solid, which was added with 25ml of ethanol, refluxed for 1 hour, cooled to room temperature, left to stand, and yellow needle-shaped crystals were precipitated , the mother liquor was further concentrated and crystallized, and a total of 7.8 g of compound 1a was obtained.
[0042] (2) Add compound 1a (0.72g, 3mmol) and p-hydrazinobenzenesulfonamide (0.67g, 3.5mmol) to 25ml of methanol, heat to reflux for 10h, follow the reactio...
Embodiment 3
[0044] Example 3 (compound 1)
[0045] The preparation method of 4-[3,5-bis(2-hydroxyphenyl)-1-(1,2,4-triazolyl)]benzenesulfonamide, the steps comprising:
[0046] (1) Add 40ml of xylene and salicylic acid (17.3g, 120mmol) into a 250ml reaction bottle, slowly add 10ml of thionyl chloride with a dropping funnel, leave it for 30min after the dropwise addition, add salicylamide (11.2g, 80mmol), reacted at room temperature for 5h, tracked the reaction by thin-layer chromatography, after the reaction was complete, evaporated the organic solvent under reduced pressure to obtain a yellow viscous solid, added 50ml of ethanol, heated to reflux for 1h, cooled to room temperature, stood still, and precipitated 7.6g Yellow powdery solid compound 1a.
[0047] (2) Add compound 1a (0.76g, 3mmol), p-hydrazinobenzenesulfonamide hydrochloride (0.65g, 3.5mmol) into 25ml of methanol, reflux for 12h, cool to room temperature, and then continue to drop to 0-5 ℃ for 1h. The precipitated solid was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com