Cefprozil suspension and preparation method thereof
A technology for cefprozil and dry suspension, which is applied in pharmaceutical formulations, medical preparations without active ingredients, medical preparations containing active ingredients, etc. and other problems, to achieve the effect of stable product quality, easy preparation process, low content of single impurities and total impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
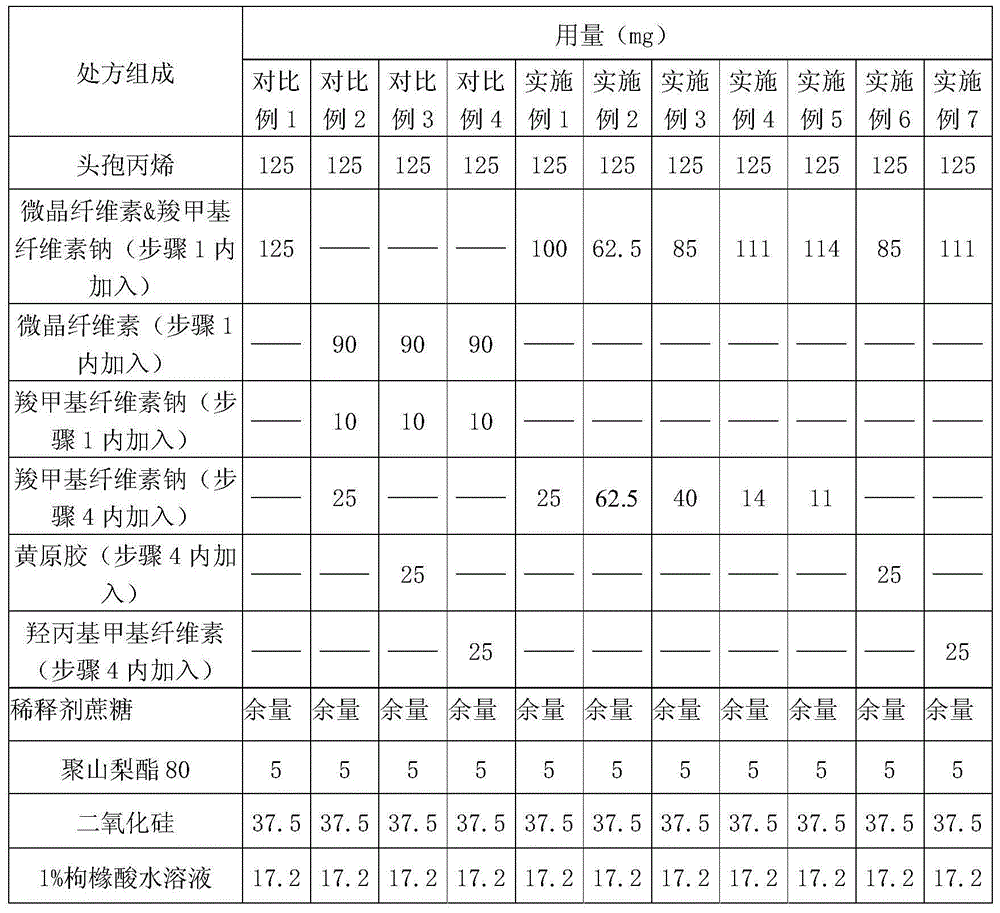
Examples
Embodiment 8-11
[0049] Embodiment 8-11 is all prepared according to the following steps:
[0050] (1) Pass the diluent, microcrystalline cellulose & sodium carboxymethyl cellulose through a 30-mesh sieve, and then pre-mix in a wet granulator for 3 minutes to obtain a pre-mixture;
[0051] (2) adding defoamer, cosolvent and wetting agent to the premixture in step 1, and carrying out wet granulation;
[0052] (3) Place the pellets obtained in step 2 in a boiling dryer through vacuum discharge, and dry at 55°C to 65°C; pass through a 20-mesh sieve for granulation, and mark it as material A;
[0053] (4) Cefprozil, suspending agent, corrective agent, essence, pigment and lubricant are mixed with material A in the manner of increasing in equal amounts, and after mixing evenly, subpackage to obtain cefprozil dry suspension.
Embodiment 8
[0054] Embodiment 8 (by preparing 1000 bag amounts)
[0055] prescription
[0056]
[0057]
Embodiment 9
[0058] Embodiment 9 (by preparing 1000 bag amounts)
[0059] prescription
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com