A kind of preparation method of Abamectin b2a
A technology of abamectin and n-butyl acetate, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of site personnel's danger, low crystal purity, and high production cost, and achieve Reduced production costs, high crystal purity, and fewer crystallization times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]A preparation method for high-purity Abamectin B2a, comprising steps as follows:
[0038] 1. Take 1500ml of abamectin B1a crystallization mother liquor after crystallization and separation of abamectin B1a in the production process of abamectin B1a and raise the temperature to 70°C, keep the temperature and distill most of the distillate under vacuum until the ointment is obtained For thick materials, the vacuum degree under vacuum conditions is 0.06Mpa;
[0039] 2. Take 450g of the ointment thick material obtained in step 1, add 1125ml of n-butyl acetate, heat up to 70°C, stir for 0.5 hours until the ointment thick material is completely dissolved, and obtain an extract, add 350ml of saturated saline, and stir at 80°C 2 hours, then stand for 15 minutes to separate layers, remove the water phase, and repeat washing twice to obtain n-butyl acetate solution;
[0040] 3. First cool the n-butyl acetate solution to 20°C quickly with a water bath, then adjust the stirring spe...
Embodiment 2
[0043] A preparation method for high-purity Abamectin B2a, comprising steps as follows:
[0044] 1. Take 1500ml of the abamectin B1a crystallization mother liquor after crystallization and separation of abamectin B1a during the production process of abamectin B1a and heat up to 80°C, keep the temperature and distill for 2h under a vacuum condition of 0.065Mpa, distill Most of the distillate is removed until the ointment thick material is obtained,
[0045] 2. Take 440g of the ointment thick material obtained in step 1, add 1100ml of n-butyl acetate, heat up to 60°C, stir for 1 hour until the ointment thick material is completely dissolved, and obtain an extract, add 430ml of saturated saline, and stir at 85°C 1 hour, and then stand for 15 minutes to separate layers, remove the water phase, and repeat washing twice to obtain n-butyl acetate solution;
[0046] 3. First, quickly cool the n-butyl acetate solution to 20°C with a water bath, then adjust the stirring speed to 10r / mi...
experiment example
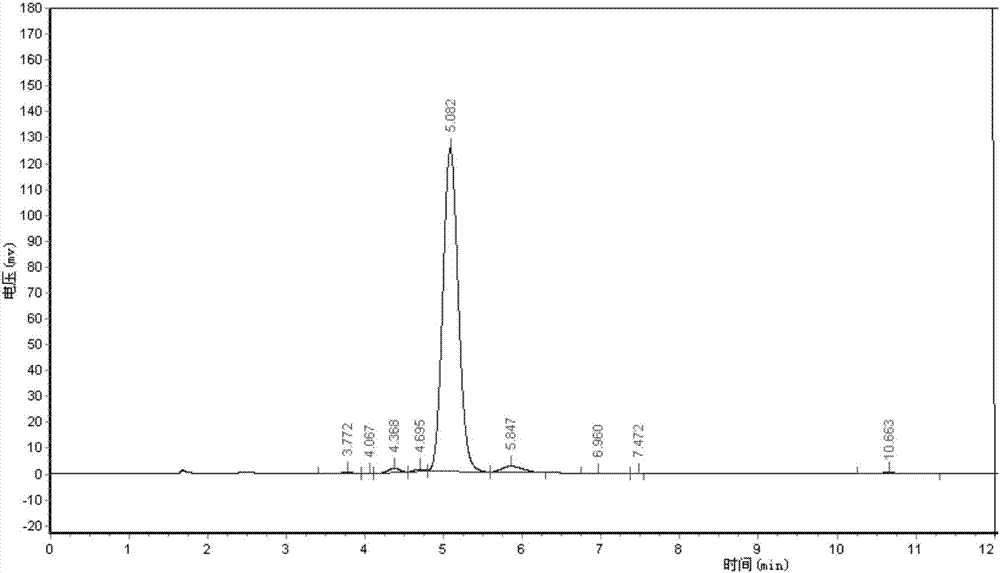
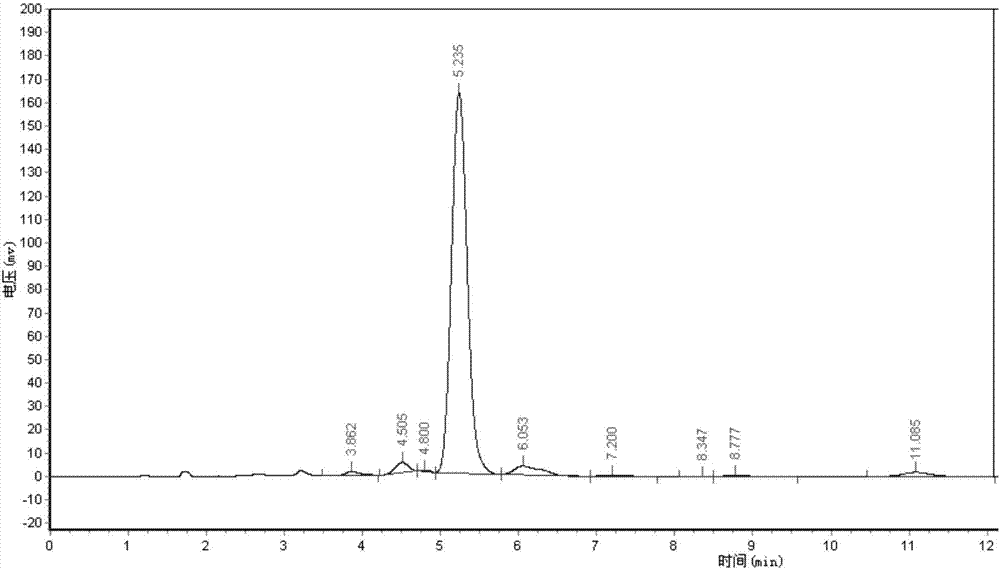
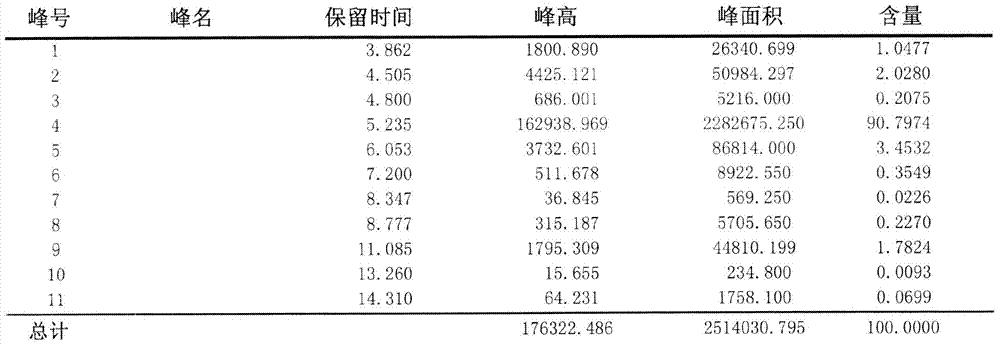
[0055] Adopt area normalization method to test the purity of embodiment 1 and comparative example 1 Abamectin B2a refined powder,
[0056] Powder detection method: high performance liquid chromatography area normalization method
[0057] Instrument: high performance liquid chromatography
[0058] Chromatographic conditions: mobile phase (methanol: water = 85:15)
[0059] Detection and wavelength: UV, 245nm
[0060] Chromatographic column (C18, 4.6mm*250mm)
[0061] Flow rate: 1ml / min
[0062] Injection volume: 20ul
[0063] Test steps: Weigh 0.025-0.030g of fine powder into a 100ml volumetric flask, dissolve with methanol, and make to volume. Draw 20ml of the solution and inject it into the high-efficiency chromatograph. According to the above chromatographic conditions, the detection time is 15 minutes, and the peak time of B2a is about 5 minutes. The purity of B2a is calculated according to the area normalization method. The purity of the Abamectin B2a fine powder of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com