Blue fluorescence N,N-bis(4-cyanophenyl)glycine zinc complex and preparation method thereof
A zinc aminoacetate, cyanophenyl technology, applied in the direction of zinc organic compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problems of restricting the large-scale application of rare earths, difficulty in rare earth mining, serious environmental pollution, etc. The effect of high yield, long service life and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
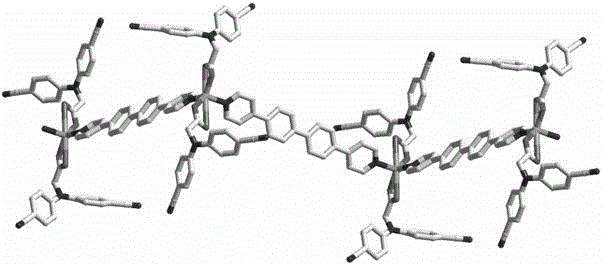
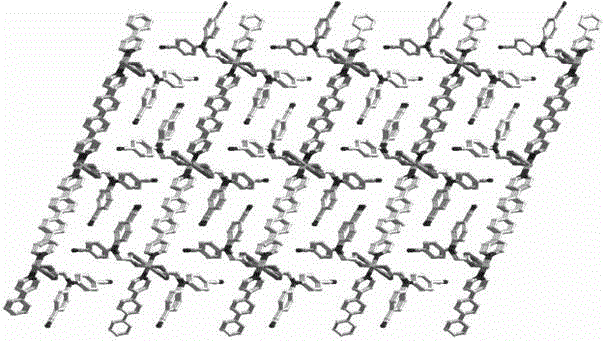
[0024] N,N-bis(4-cyanophenyl)aminoacetic acid (0.05 mmol, 14.0 mg), 4,4'-bispyridylbiphenyl ligand (0.05 mmol, 15.4 mg) and zinc nitrate (0.15 mmol, 28.4mg) was dissolved in water (8 mL) and stirred for 5 minutes, then sealed in a 25mL hydrothermal reaction kettle. The reaction mixture was then heated to 80°C at 10°C per hour, maintained at this temperature for 3 days, and then cooled to room temperature to obtain a colorless blocky crystal. The single crystal was separated, washed and dried in turn to obtain the target Product, about 75% yield.
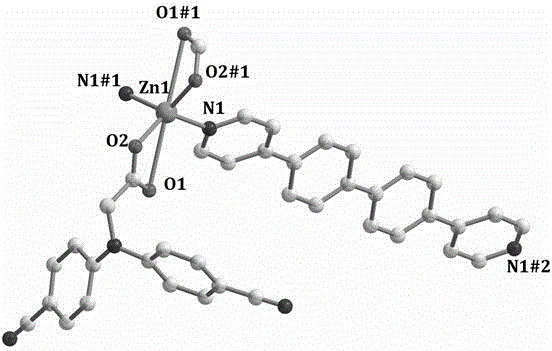
[0025] N,N-bis(4-cyanophenyl)aminoacetic acid zinc complex structure determination of the present embodiment:
[0026] (1) Determination of the crystal structure of the complex
[0027] Under the microscope, single crystals of appropriate size are selected for X-ray diffraction experiments at room temperature. On the Xcalibur, Eos, Gemini diffractometer, the diffraction data were collected in the φ–ω mode using Mo–Ka rays (l = 0.7...
Embodiment 2
[0035] N,N-bis(4-cyanophenyl)aminoacetic acid (0.1 mmol, 27.7 mg), 4,4'-bispyridylbiphenyl ligand (0.05 mmol, 15.4 mg) and zinc nitrate (0.15 mmol, 28.4mg) was dissolved in water (10 mL) and stirred for 5 minutes, then sealed into a 25ml hydrothermal reaction kettle. The reaction mixture was then heated to 80°C at 10°C per hour, maintained at this temperature for 3 days, and then cooled to room temperature to obtain a colorless blocky crystal. The single crystal was separated, washed and dried in turn to obtain the target Product, about 70% yield.
Embodiment 3
[0037] N,N-bis(4-cyanophenyl)aminoacetic acid (0.05 mmol, 14.0 mg), 4,4'-bispyridylbiphenyl ligand (0.1 mmol, 30.8 mg) and zinc nitrate (0.15 mmol, 28.4mg) was dissolved in water (8 mL) and stirred for 5 minutes, then sealed into a 25ml hydrothermal reaction kettle. Then the reaction mixture was heated at 10 °C per hour to 100 °C, maintained at this temperature for 3 days, and then lowered to room temperature to obtain a colorless block crystal, which was separated, washed and dried successively to obtain The target product, the yield is about 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com