Use of CD133<+> endothelial precursor cells
A technology of endothelial precursor cells and uses, which can be used in medical preparations containing active ingredients, skin diseases, metabolic diseases, etc., and can solve problems such as large trauma, poor wound healing, and intolerable time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] The selection and grouping of the clinical cases of embodiment 1
[0116] 1.1 Take 30 patients with diabetic foot, the inclusion criteria:
[0117] (1) History of diabetes > 10
[0118] (2) Age ≤ 75 years old
[0119] Lower extremity ischemia 2 ≤ Rutherford grade (Table 1) ≤ 4
[0120] (3) Ankle-brachial index (ABI)<0.7
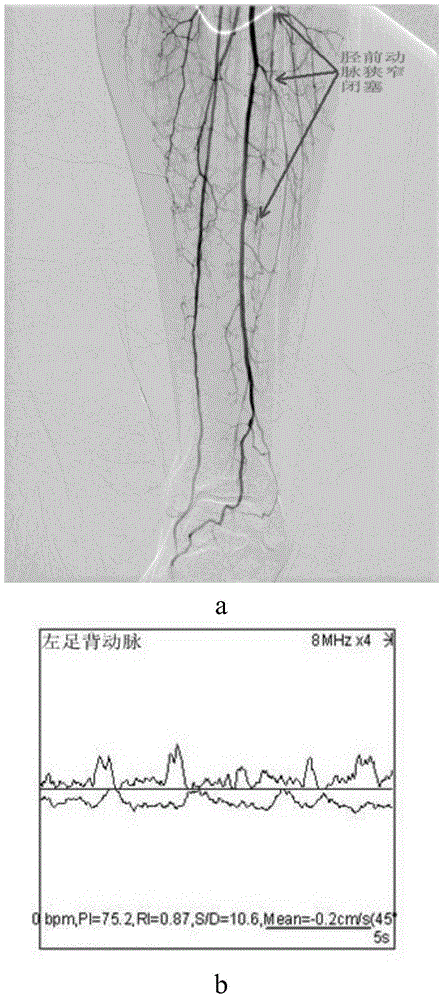
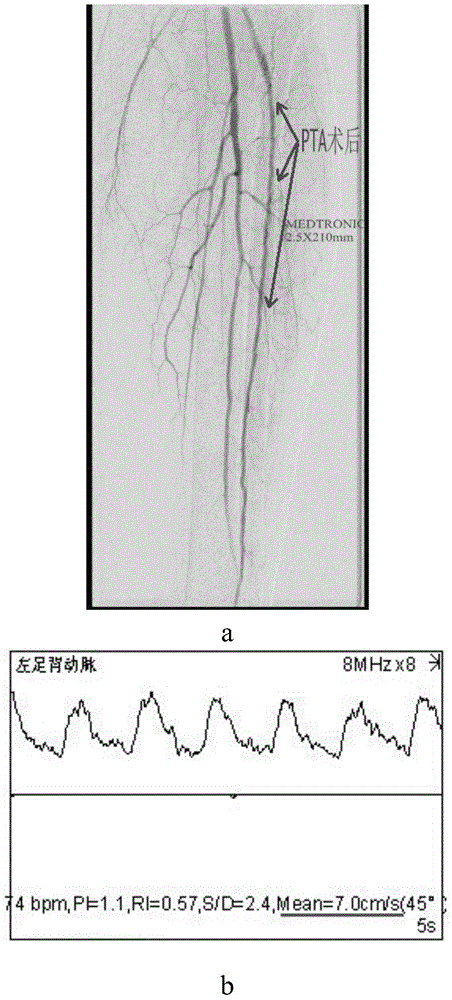
[0121] (4) The anterior tibial, posterior tibial, and peroneal arteries at the distal end of the popliteal artery of the affected limb were identified by angiography (DSA), and one or two arteries were narrowed by more than 50%.
[0122] 1.2 The exclusion criteria are met when one of the following situations occurs:
[0123] (1) Hemoglobin <10mg / dl
[0124] (2) Creatinine clearance rate <30ml / min
[0125] (3) Have received stem cell therapy before
[0126] (4) Suffering from mental illness
[0127] (5) During the treatment follow-up period, the 2hr postprandial blood glucose monitoring exceeds 11.1mmol / L for 4 consecutive weeks
[0128] (6) Du...
Embodiment 2
[0139] Example 2 CD133 + Acquisition of EPCs and combined treatment plan
[0140] 2.1. For all the subjects in Example 1, 100 ml of venous blood will be collected through the central vein and anticoagulated with heparin.
[0141] The blood of subjects in group A will be handed over to the GMP-certified East China Stem Cell Bank laboratory for CD133 testing within 24 hours according to general method 1. + After the magnetic bead sorting of EPCs, endotoxin and other routine blood product safety tests are passed, aseptic packaging (50ml suspension, cell volume ≥ 2×10 7 ), stored and transported at a constant temperature of 4°C. The blood of subjects in group B will be used for laboratory research.
[0142] 2.2. Basic treatment of diabetes
[0143] Blood glucose control plan and related standardized routine treatment will be formulated by endocrinology specialist, and PTA / CD133 will be tested before blood collection + After EPCs treatment, the 2hr postprandial blood glucose w...
Embodiment 3
[0159] Embodiment 3 follows up a case by regular visits to the patient in embodiment 2 result
[0160] Follow-up arrangements are shown in Table 2
[0161] Table 2
[0162]
[0163] The basic information of the patients is shown in Table 3: 14 cases, type II diabetes
[0164] table 3
[0165] age 55-83 years old
[0166] 6 male cases (1 case 2 times), 7 female cases Before the follow-up visit 2012.12.18-2013.06.30 average age 69.77±9.66 years old Lower Extremity Pain Relief 13 / 14 Lower extremity cold improved 13 / 14 ulcer healing 2 / 3 ABI improvement 12 / 14 limb salvage 14 / 14
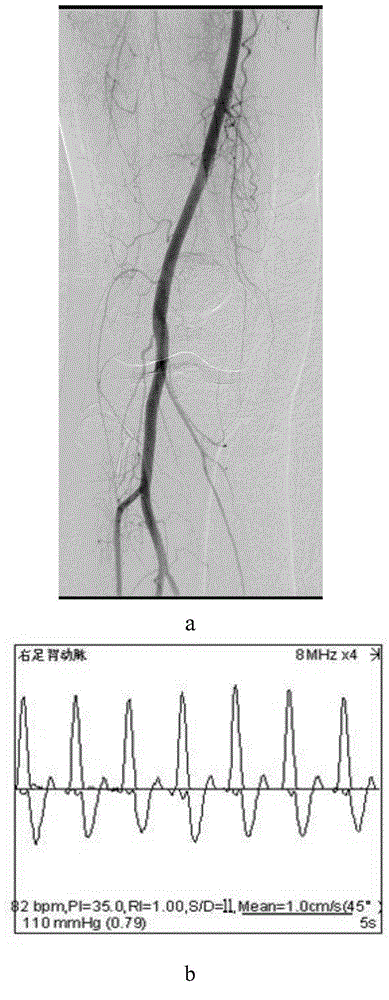
[0167] For specific results, see Figure 5-Figure 7 :
[0168] in, Figure 5 It shows that the overall ABI index and blood flow velocity of the patients after the operation are better than before. Figure 6 a-c shows that the postoperative follow-up is compared with the preoperative, and the patient's red blood cell, le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com