Method for measuring fat-soluble platinum complex and preparation related substances thereof
A technology for related substances and preparations, which is applied in the field of high-performance liquid chromatography to detect related substances in Miplatin raw materials and freeze-dried powder injection, and can solve the problems of difficulty in separating impurity A peak and solvent peak, impurity A cannot be effectively detected, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel as filler; mobile phase: A phase: methanol: ethanol (7:3), B phase: methanol: ethanol: water (7:3:40 ); flow rate 1.0mL / min; injection volume 20μL; column temperature 40°C; detection wavelength 210nm. The number of theoretical plates is not less than 2000 based on impurity A. Gradient times are as follows:
[0100] time (min)
A(v%)
B(v%)
0
85
15
[0101] 15
85
15
20
100
0
50
100
0
55
85
15
65
85
15
[0102] Determination method Take about 10mg of miplatin raw material, weigh it accurately, put it in a 10mL measuring bottle, add an appropriate amount of chromatographic ethanol and ultrasonically dissolve it, dilute it with ethanol to the mark, shake well, and filter it as the test solution. Take 20 μL each of the test solution, inject it into the liquid chrom...
Embodiment 2
[0123] Mobile phase: Phase A: methanol: ethanol (95:5), phase B: methanol: ethanol: water (95:5:400);
[0124] Gradient times are as follows:
[0125] time (min)
A(v%)
B(v%)
0
85
15
15
85
15
20
100
0
50
100
0
55
85
15
65
85
15
[0126] Other chromatographic conditions are the same as in Example 1.
[0127] Determination method Same as implementation 1.
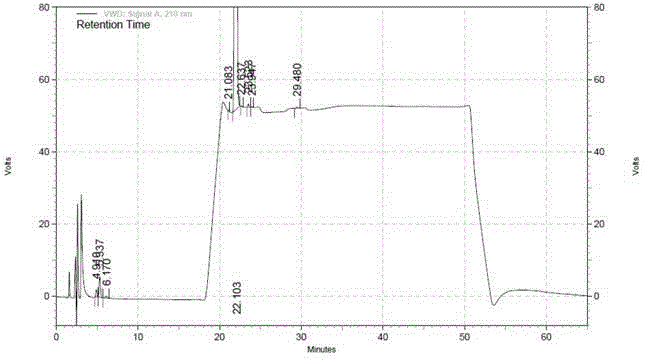
[0128] The separation of the known impurity A and other unknown impurities in the rice platinum raw material by this method is greater than 1.5, which meets the requirements.
[0129] method validation
[0130] A. System suitability experiment:
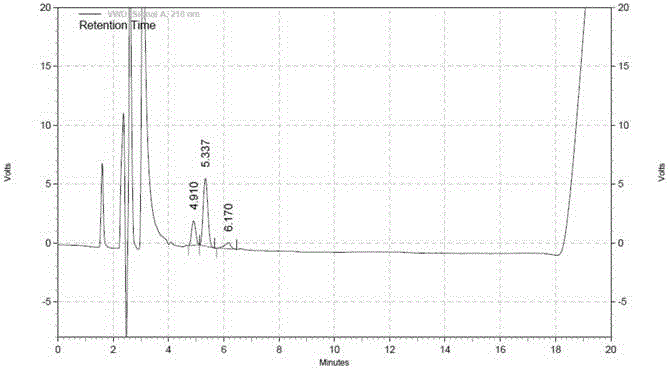
[0131] Take the impurity A reference substance, according to the above chromatographic conditions, continuously inject 5 needles, the RSD of the impurity A peak area in the reference substance solution is 5.87%, the number of theoretical plates is 3491, and the system ...
Embodiment 3
[0149] Mobile phase: Phase A: methanol: ethanol (75:25), phase B: methanol: ethanol: water (75:25:400);
[0150] Gradient times are as follows:
[0151] time (min)
A(v%)
B(v%)
0
90
10
15
90
10
20
100
0
50
100
0
55
90
10
65
90
10
[0152] Other chromatographic conditions are the same as in Example 1.
[0153] The assay method is the same as that in Embodiment 1, except that the test sample is the freeze-dried powder of miplatin.
[0154] The separation of the known impurity A and other unknown impurities in the lyophilized powder of miplatin by this method is greater than 1.5, which meets the requirements.
[0155] method validation
[0156] A. System suitability experiment:
[0157] Take the impurity A reference substance, according to the above chromatographic conditions, continuously inject 5 needles, the RSD of the impurity A peak area in the reference subst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com