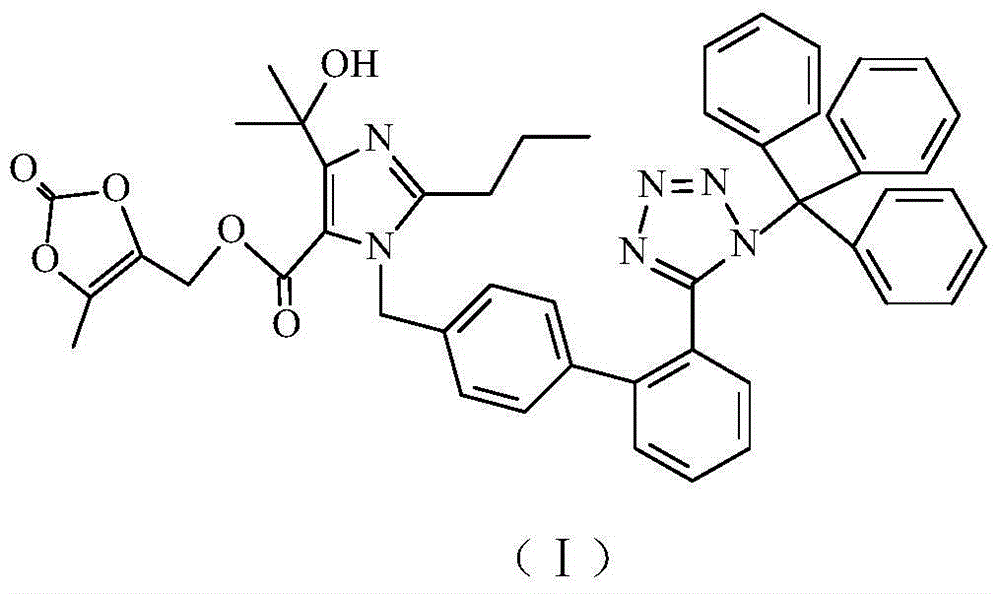
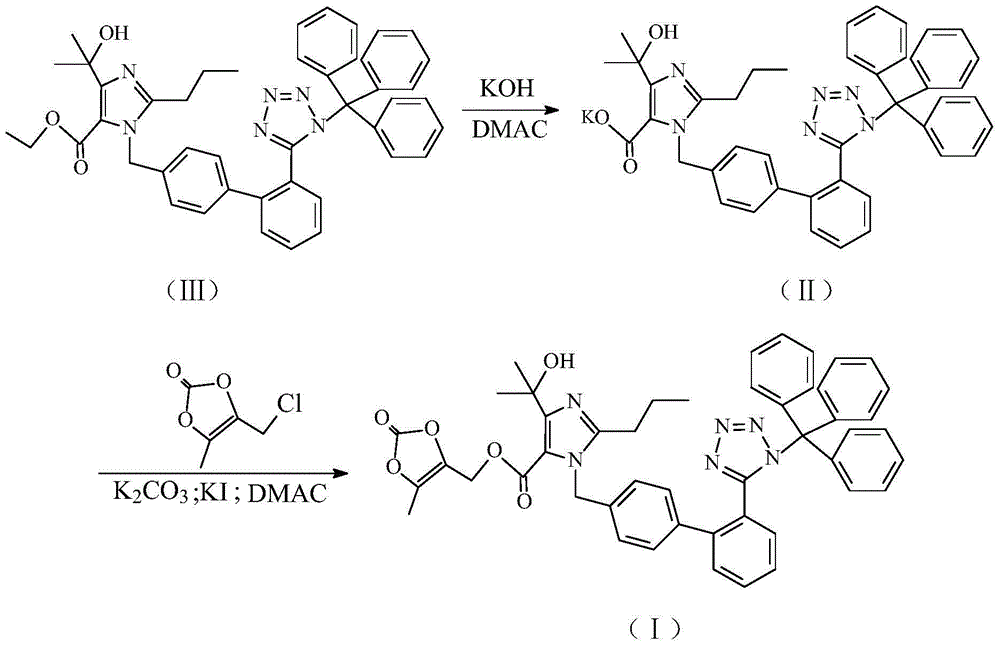
Preparation method of olmesartan intermediate
A technology of olmesartan medoxomil and intermediates, applied in the field of drug synthesis, can solve the problems of difficult industrial production, difficult removal of solvents, poor product quality, etc., and achieve the effects of easy control of process conditions, good product quality and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033](1) Add 160kg of N,N-dimethylacetamide in a 500L glass-lined reactor equipped with devices such as stirrer, thermometer, vacuum pressure gauge, etc., add 40kg of compound (Ⅲ) and 4.0kg of potassium hydroxide powder under stirring, Close the feeding port, replace the air with nitrogen and keep the pressure ≥ 0.01MPa, control the temperature of the reaction mixture under stirring, keep stirring at 35°C for 5 hours, then add 12kg of anhydrous potassium carbonate powder and 6.0kg of potassium iodide to the reaction kettle, The internal temperature of the reaction mixture was lowered to 0-5°C, and 16 kg of 4-chloromethyl-5-methyl-1,3-dioxol-2-one was added. After replacing the air with nitrogen, in the presence of nitrogen under positive pressure, raise the internal temperature of the reaction solution to 30-35°C within 1 hour. Transfer to a 2000L extraction kettle, add 480kg of ethyl acetate, wash with 1000kg of 10% sodium chloride aqueous solution three times, control the t...
Embodiment 2
[0037] (1) Add 280kg of N,N-dimethylacetamide to a 500L glass-lined reactor equipped with a stirrer, thermometer, vacuum pressure gauge, etc., add 40kg of compound (Ⅲ) and 12kg of potassium hydroxide powder under stirring, and close the At the feeding port, replace the air with nitrogen and keep the pressure ≥ 0.01MPa, control the temperature of the reaction mixture under stirring, keep stirring at 15°C for 3 hours, then add 4kg of anhydrous potassium carbonate powder and 2kg of potassium iodide to the reaction kettle, and then react The internal temperature of the mixed solution was lowered to 0-5°C, and 10 kg of 4-chloromethyl-5-methyl-1,3-dioxol-2-one was added. After replacing the air with nitrogen, in the presence of nitrogen under positive pressure, raise the internal temperature of the reaction solution to 45-50°C within 1 hour, and after holding the heat for 1 hour, reduce the internal temperature of the reaction mixture to 10°C, and the reaction solution Transfer to a...
Embodiment 3
[0041] (1) Add 200kg of N,N-dimethylacetamide in a 500L glass-lined reactor equipped with devices such as stirrer, thermometer, vacuum pressure gauge, etc., add 40kg of compound (Ⅲ) and 8.0kg of potassium hydroxide powder under stirring, Close the feeding port, replace the air with nitrogen and keep the pressure ≥ 0.01MPa, control the temperature of the reaction mixture under stirring and keep stirring at 20°C for 4 hours, then add 8.0kg of anhydrous potassium carbonate powder and 4.0kg of potassium iodide to the reaction kettle , the internal temperature of the reaction mixture was lowered to 0-5° C., and 12 kg of 4-chloromethyl-5-methyl-1,3-dioxol-2-one was added. After replacing the air with nitrogen, in the presence of nitrogen under positive pressure, raise the internal temperature of the reaction solution to 35-40°C within 1 hour. Transfer to a 2000L extraction kettle, add 600kg of ethyl acetate, wash with 1120kg of 10% sodium chloride aqueous solution three times, contr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com