A method for recovering lithium resources from lithium-ion-containing solutions using lithium ion carriers
A lithium ion and lithium recovery technology, applied in the field of lithium resource extraction, can solve problems such as environmental pollution, complicated process, equipment corrosion, etc., and achieve the effect of reducing recycling costs, simple process and efficient recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
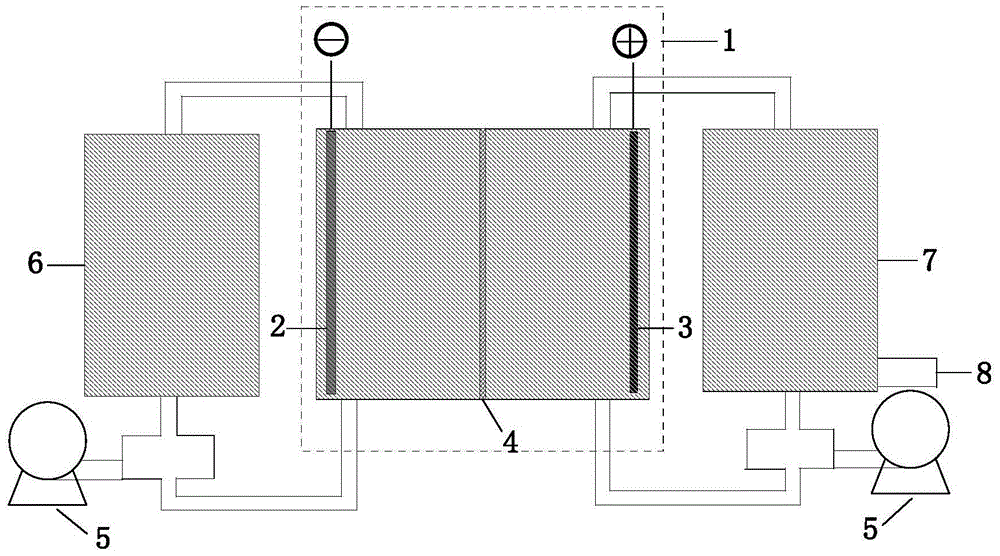
[0035] With 100 g LiCoO 2 As a lithium ion carrier in a lithium-rich state, 316L stainless steel is made into a cylindrical shape (diameter 8cm, height 6cm). As an anode, the lithium ion carrier is placed in a straight cylinder and stirred with magnetic force at the bottom of the reaction vessel to ensure that LiCoO 2 The effective contact with the electrodes is in a suspended state in the ring-shaped base, and the cathode is a nickel foam cylindrical cathode (diameter 5cm, height 6cm) separated by diaphragm paper. The anode and cathode solutions of the electrolytic cell are filled with the same 0.5mol / L LiCl solution, connected to an external DC power supply, and electrolyzed at a constant current of 1A (current density: 10mA / g) for 16 hours to make LiCoO 2 Lithium ion carriers transformed into a lithium-poor state.
[0036] The lithium ion carrier obtained in the lithium-poor state above is used as a carrier for absorbing lithium ions in the salt lake brine solution. At th...
Embodiment 2
[0041] Spinel LiMn after delithiation by pre-acidification 2 o 4 As a lithium ion carrier in a lithium-poor state, the pre-acidification refers to adding 10 grams of LiMn 2 o 4 Stir continuously in 2 liters of 0.2 mol / L HCl solution to make it fully contact, and after reacting for 12 hours, filter, wash and dry to obtain a lithium ion carrier in a lithium-poor state. (Be prior art) it is made into electrode, and the weight percent content of four components of electrode is as follows:
[0042] The lithium ion carrier is spinel LiMn after pre-acidification and delithiation 2 o 4 : 51.3%;
[0043] The conductive material is expanded graphite: 8.8%;
[0044] Carrier material nickel foam: 37.8%;
[0045] Binder PTFE: 2.1%.
[0046] The counter electrode is a capacitive carbon mixed in PTFE to make an auxiliary electrode. Place the above-mentioned electrode sheet containing the carrier of lithium ions in a lithium-deficient state on the cathode carrier of the electrolyzer,...
Embodiment 3
[0048] Commercially available LiCoO 2 It is a carrier of lithium ions in a lithium-rich state, and it is made into an electrode. The weight percentages of the four components of the electrode are as follows:
[0049] The lithium ion carrier is LiCoO 2 : 49.4%;
[0050] The conductive material is expanded graphite: 9.6%;
[0051] Carrier material nickel foam: 38.8%;
[0052] Binder PTFE: 2.2%.
[0053] The recovery process of lithium ions is as follows:
[0054] (1) 100 g of LiCoO 2 The lithium ion carrier electrode made according to the above ratio is used as an anode, the counter electrode is a capacitor carbon mixed in PTFE to make an auxiliary electrode, and the reference electrode is an SCE electrode. The cathode chamber is fed with 0.5 liter of 0.5mol / LMgSO to be recovered 4 and 0.5mol / L Li 2 SO 4 The mixed solution is catholyte. The anode chamber is fed with 1 liter of 1mol / L Na 2 SO 4 And 0.005mol / LLiOH solution is the anolyte.
[0055] In relative to LiCoO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com