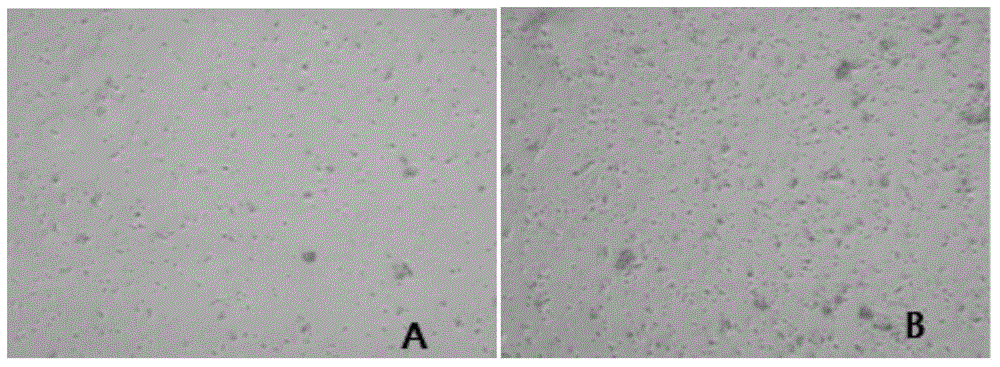
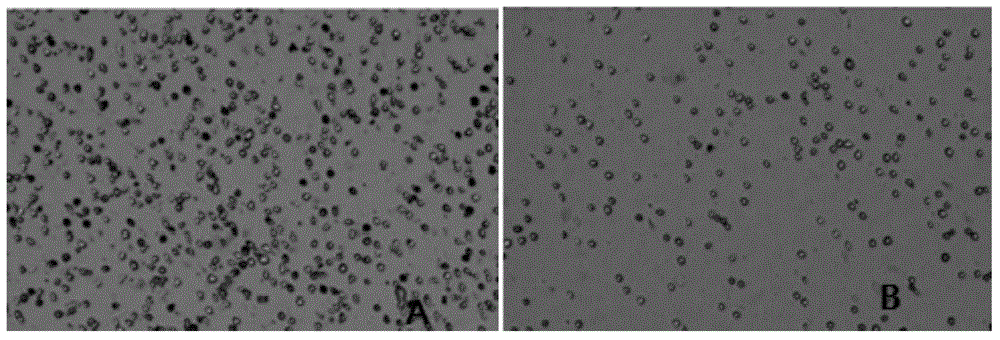
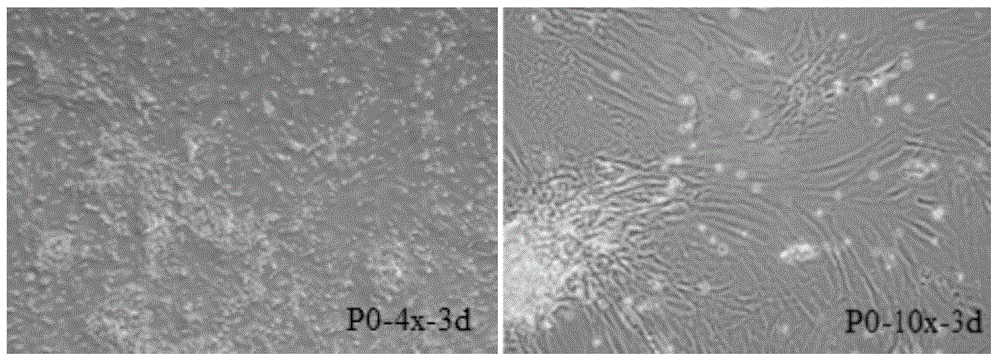
Primary isolation culture method of adipose-derived stem cells
A technology for the separation and cultivation of adipose stem cells, which is applied in the direction of animal cells, vertebrate cells, bone/connective tissue cells, etc., can solve the problems of unsatisfactory separation and culture methods of ADSCs, slow proliferation speed, and cytotoxicity, and achieve favorable scale Chemical production and preparation, stable enzymatic hydrolysis conditions, and high cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Let the adipose tissue containing interstitial fluid stand still, and after the adipose tissue and the liquid are naturally separated, suck up the liquid below with a 10mL pipette; use a 50mL syringe to draw an equal volume of PBS and add it to the adipose tissue, cover the bottle cap and shake the adipose tissue repeatedly tissue, let it stand for 5 minutes; use a 25mL pipette to discard the solution under the adipose tissue (repeat once).
[0028] 2) Transfer the adipose tissue to a 50mL centrifuge tube with a disposable pipette; add an equal volume of type I collagenase and trypsin mixture (0.25% and 0.05% by mass), and shake the bottle upside down after sealing. Mix well. Transfer to a constant temperature air bath shaker at 37°C, digest at 200R for 50min
[0029] 3) Add 1 mL of FBS to each tube, cap the bottle and shake well, centrifuge at 1500rpm / min for 5min; discard the supernatant after centrifugation.
[0030] 4) According to the ratio of fat volume: 10% ...
Embodiment 2
[0034] 1) Let the adipose tissue containing interstitial fluid stand still, and after the adipose tissue and the liquid are naturally separated, suck up the liquid below with a 10mL pipette; use a 50mL syringe to draw an equal volume of PBS and add it to the adipose tissue, cover the bottle cap and shake the adipose tissue repeatedly tissue, let it stand for 5 minutes; use a 25mL pipette to discard the solution under the adipose tissue (repeat once).
[0035] 2) Transfer the adipose tissue to a 50mL centrifuge tube with a disposable pipette; add an equal volume of type I collagenase and trypsin mixture (0.05% and 0.1% by mass), and shake the bottle upside down after sealing. Mix well. Transfer to a constant temperature air bath shaker at 37°C, digest at 100R for 30min
[0036] 3) Add 1 mL of FBS to each tube, cap the bottle and shake well, centrifuge at 1000rpm / min for 3min; discard the supernatant after centrifugation.
[0037] 4) According to the ratio of fat volume: 10% F...
Embodiment 3
[0041] 1) Let the adipose tissue containing interstitial fluid stand still, and after the adipose tissue and the liquid are naturally separated, suck up the liquid below with a 10mL pipette; use a 50mL syringe to draw an equal volume of PBS and add it to the adipose tissue, cover the bottle cap and shake the adipose tissue repeatedly tissue, let it stand for 5 minutes; use a 25mL pipette to discard the solution under the adipose tissue (repeat once).
[0042] 2) Transfer the adipose tissue to a 50mL centrifuge tube with a disposable pipette; add an equal volume of type I collagenase and trypsin mixture (0.3% and 0.01% by mass), and shake the bottle upside down after sealing. Mix well. Transfer to a constant temperature air bath shaker at 37°C, digest at 250R for 60min
[0043] 3) Add 1 mL of FBS to each tube, cap the bottle and shake well, centrifuge at 1500rpm / min for 10min; discard the supernatant after centrifugation.
[0044] 4) According to the ratio of fat volume: 10% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com