A method for rapidly preparing long-chain branched polylactic acid resin and polylactic acid resin prepared therefrom
A technology of long-chain branched polylactic acid and polylactic acid resin, applied in the field of polylactic acid resin, can solve the problem that it is difficult to obtain a product with a larger molecular weight in an amount of oxazoline compound, the product molecular weight and the degree of branching are difficult, and the melt strength is difficult. Problems such as not greatly improved, to achieve the effect of significant branching effect, effective branching effect, high molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Two-step functional group reaction for rapid preparation of long-chain branched polylactic acid
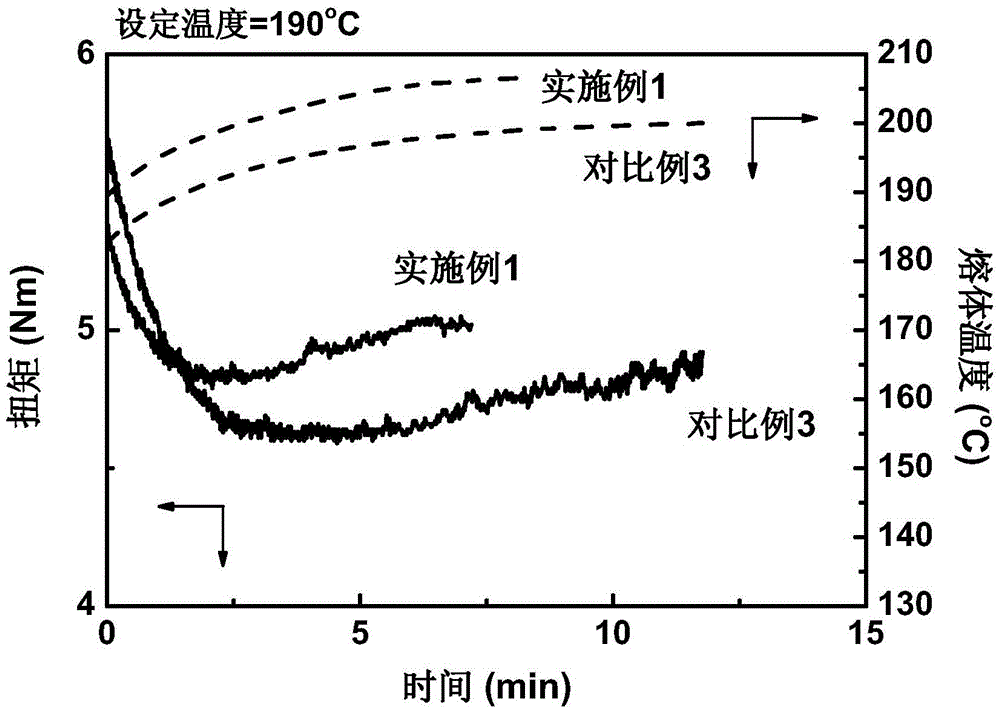
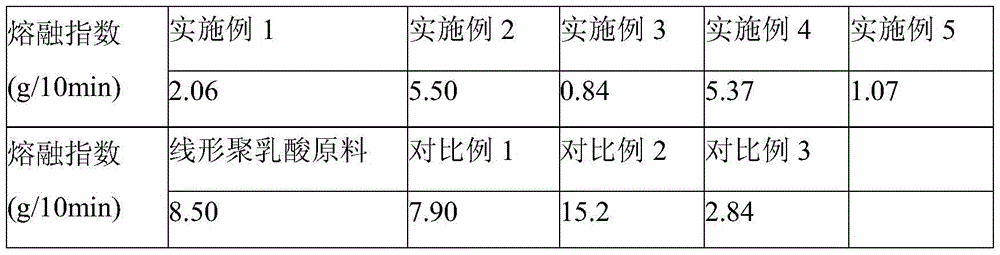
[0028]Add 100 parts by weight of polylactic acid resin to an internal mixer with a rotation speed of 20 rpm at 190°C, and then add 0.15 parts of 1,3,5-trimethyl-2,4,6-tris(3 ,5-di-tert-butyl-4-hydroxybenzyl)benzene, 0.37 parts of pyromellitic dianhydride, 1 part of 2,2'-(1,4-phenylene)bisoxazoline and three 0.1 part of phenylphosphine, mixed for 2 minutes; then the internal mixer was raised to 60 rpm, and blended until the reaction was completed, and stopped after about 7 minutes to obtain long-chain branched polylactic acid resin. The results of the melt index (190°C, 2.16kg load) of the obtained reaction product material according to the ASTM D1238 standard are shown in Table 1, and the torque and temperature curves in the internal mixer are shown in Table 1. figure 1 .
Embodiment 2
[0030] Two-step functional group reaction for rapid preparation of long-chain branched polylactic acid
[0031] 100 parts by weight of polylactic acid resin, 0.15 parts of 1,3,5-trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)benzene, 0.37 parts Pyromellitic dianhydride, 0.41 parts of 2,2'-(1,4-phenylene) bisoxazoline and 0.1 part of triphenylphosphine, added at 200°C to a twin-screw extruder with a rotation speed of 200rpm Machine, direct extrusion to obtain long-chain branched polylactic acid resin. The melt index test results are shown in Table 1.
Embodiment 3
[0033] Two-step functional group reaction for rapid preparation of long-chain branched polylactic acid
[0034] 100 parts by weight of polylactic acid resin, 0.15 parts of 1,3,5-trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)benzene, 0.37 parts Pyromellitic dianhydride, 2.46 parts of 2,2'-(1,4-phenylene) bisoxazoline and 0.08 parts of triphenylphosphine, added at 200°C to a twin-screw extruder with a rotation speed of 200rpm Machine, direct extrusion to obtain long-chain branched polylactic acid resin. The melt index test results are shown in Table 1,
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com