Metallocene complex, preparation method thereof and catalyst composition
一种茂金属配合物、缩酮的技术,应用在茂金属、化学仪器和方法、含周期表第3/13族元素的化合物等方向,能够解决研究未见报道等问题,达到改变电子效应、增加活性、高插入率的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The present invention provides a method for preparing the metallocene complex represented by the above formula (I), comprising:
[0064] When the metallocene complex X shown in formula (I) 1 with X 2 When each is independently a C1-C20 alkoxy group, the metallocene complex represented by the formula (I) is prepared according to the following steps: under the condition of inert gas protection, the cyclopentadiene compound represented by the formula (II) The alkenyl ligand is reacted with the rare earth compound in the first organic solvent to obtain the metallocene complex represented by formula (I).
[0065]
[0066] Wherein, Ln is scandium (Sc), yttrium (Y) and one of the lanthanide fourteen elements with atomic number 57-71;
[0067] R 1 , R 2 , R 3 , R 4 with R 5 each independently selected from H, C1-C20 alkyl, C1-C20 alkyl containing acetal, C1-C20 alkyl containing ketal, C1-C20 alkyl containing ether group, C1-C20 Alkenyl group, C1-C20 alkenyl group con...
Embodiment 1
[0105] Under the condition of nitrogen protection, 0.3g (1.56mmol) of thiophene-fused cyclopentadienyl ligand 1 was dissolved in 8ml of n-hexane, and added dropwise to the solution containing 0.7g (1.56mmol) of Sc(CH 2 SiMe 3 ) 3 (thf) 2 In n-hexane solution, react for 12h, concentrate the reaction solution, and recrystallize to obtain 0.57g of pale yellow thiophene-fused scandium-type scandium complex (I-1), molecular formula C 22 h 41 OSScSi 2 , the productive rate is 75%, and the reaction formula is as follows:
[0106]
[0107] The elemental analysis (%) of the scandium alkyl complex (I-1) obtained in Example 1 was analyzed, and the elemental analysis (%) result was: C58.52; H8.85.
Embodiment 2
[0109] Ligand 1 in Example 1 was replaced by ligand 2, and the rest of the steps were the same to obtain a thiophene-fused scandium-type complex (I-2), molecular formula C 24 h 45 OSScSi 2 , the reaction formula is as follows:
[0110]
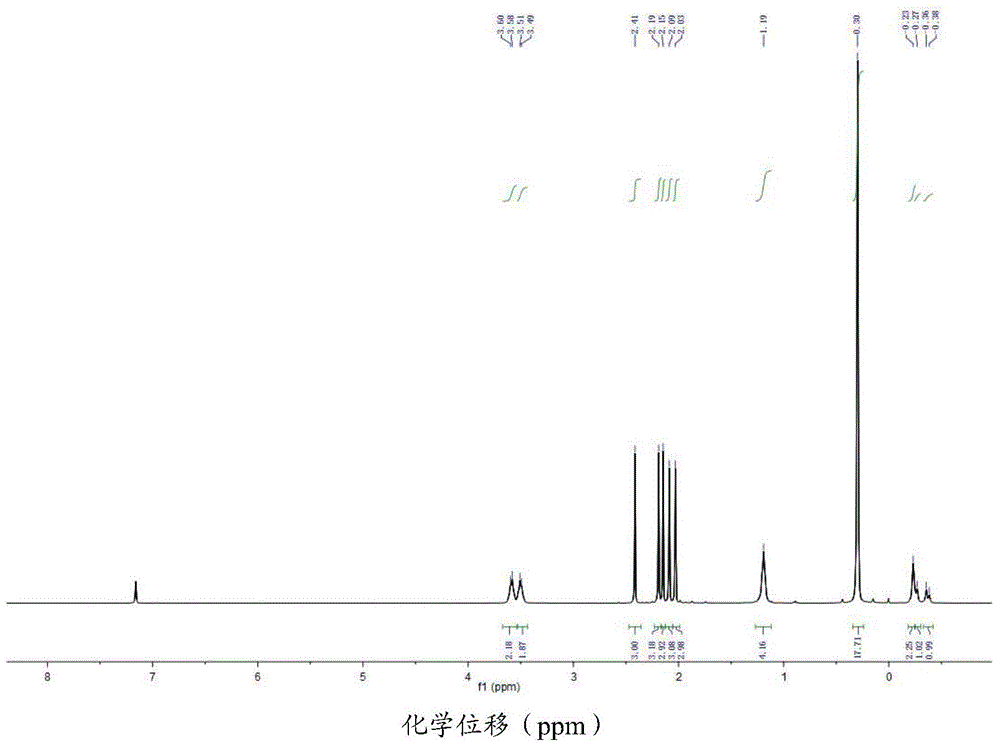
[0111] The cenecene-type scandium alkyl complex (I-2) obtained in Example 2 is analyzed by nuclear magnetic resonance, and its hydrogen nuclear magnetic resonance spectrum is obtained, as shown in figure 1 shown. The NMR results are:
[0112] 1 H NMR (C 6 D. 6 ,25℃): δ3.59(br s, 2H, THF), 3.50(br s, 2H, THF), 2.41(s,3H), 2.19(s,3H), 2.15(s,6H), 2.09( s,1H),1.19(br s,4H),0.30(s,18H,CH 2 SiMe 3 ),-0.23(br s,2H,CH 2 SiMe 3 ),-0.28(d,J=0.08Hz,1H,CH 2 SiMe 3 ),-0.37(d,J=0.08Hz,1H,CH 2 SiMe 3 )ppm.
[0113] 13 CNMR (C 6 D. 6 ,25℃): δ133.31, 130.43, 126.24, 124.77, 121.80, 110.18, 109.12, 71.54, 24.96, 24.77, 13.87, 13.30, 13.03, 12.68, 12.64, 12.28, 4.34ppm.
[0114] The elemental analysis (%) of the scandium alkyl complex (I-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com