Method for preparing carbonyl ferrocene condensed 2-amino-5-substituted-1,3,4-thiadiazole
A technology of acyl ferrocene and acetyl ferrocene, applied in chemical instruments and methods, metallocene, organic chemistry, etc., can solve the problems of difficult to achieve mass production, complicated post-processing process, and high reaction temperature, and achieve the effect Good, short response time, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Add 0.001mol ferroceneformaldehyde, 0.001mol 2-amino-5-methyl-1,3,4-thiadiazole and 0.0012mol p-toluenesulfonic acid to a dry mortar, and grind at room temperature until the raw materials react complete (reaction monitored by TLC), the time is 15 min, and then it is kept in an oven at 50° C. for 30 min.
[0034] 2) Then cool it down to room temperature and add absolute ethanol, filter the mixture with suction to remove the catalyst p-toluenesulfonic acid, and concentrate the filtrate to dryness under reduced pressure to obtain the crude product. The crude product is recrystallized with absolute ethanol to obtain formyl ferrocene 2-amino-5-methyl-1,3,4-thiadiazole.
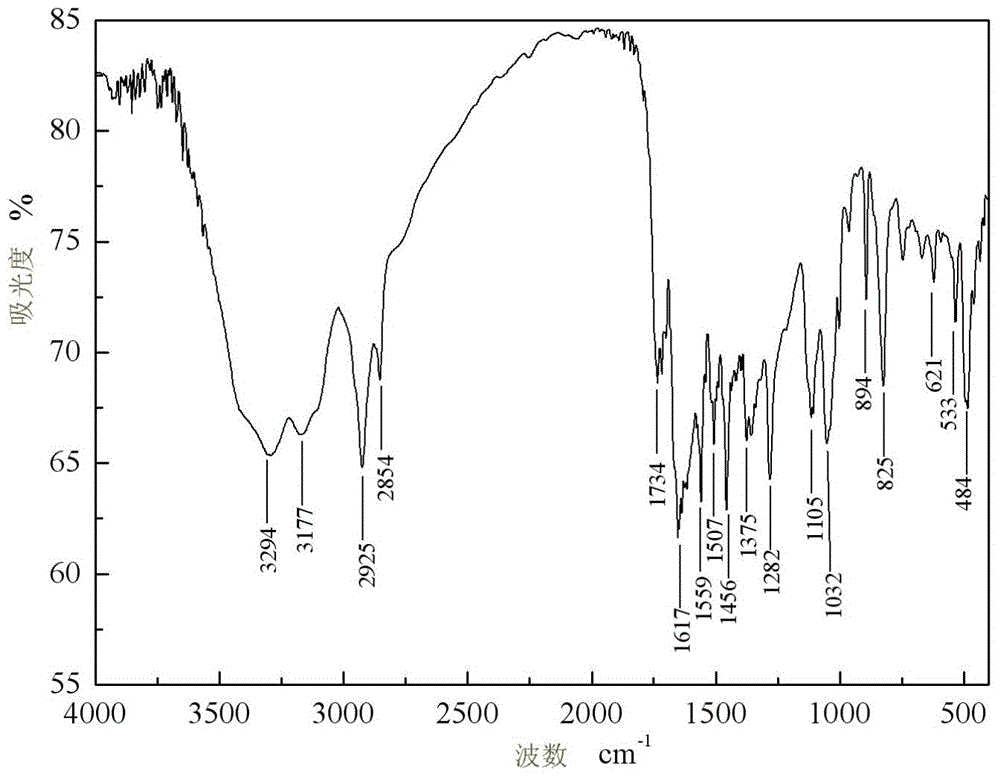
[0035] IR (KBr, v / cm -1 ): 3555, 3473, 2960, 2933, 1618, 1455, 1378, 1377, 1196, 1196, 1074, 669, 479;
[0036] 1 H-NMR: 4.820 (m, 2H, replace the H on the ortho position of C=N on the alocene ring); 4.752 (m, 2H, replace the H on the alocene ring and the C=N meta-position); 2.505 (vs, 6H, DMSO-d б s...
Embodiment 2
[0038] 1) Add 0.001mol acetylferrocene, 0.001mol 2-amino-1,3,4-thiadiazole and 0.0012mol p-toluenesulfonic acid to a dry mortar, and grind at room temperature until the reaction of the raw materials is complete (monitored by TLC reaction), the time is 20min, and then it is kept in an oven at 50°C for 30min.
[0039] 2) Then cool it down to room temperature and add absolute ethanol, filter the mixture with suction to remove the catalyst p-toluenesulfonic acid, and concentrate the filtrate to dryness under reduced pressure to obtain the crude product. The crude product is recrystallized with absolute ethanol to obtain 2-amino-1,3,4-thiadiazole acetyl ferrocene.
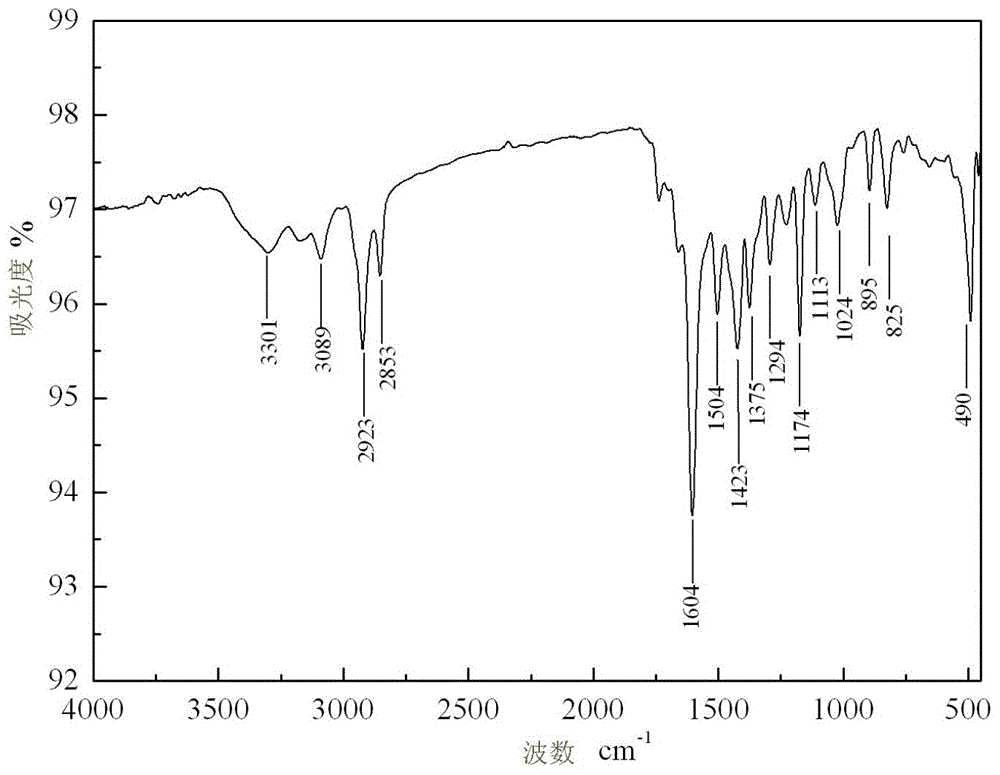
[0040] Depend on figure 1 Visible, 3301, 3089cm -1 It is the strong absorption peak of C-H stretching vibration on ferrocene; 1504, 1423cm -1 It is the strong peak of C=N stretching vibration absorption; 825cm -1 It is the C-H stretching vibration absorption weak peak on ferrocene; 490cm -1 Absorb weak peaks for N-...
Embodiment 3
[0043] 1) Add 0.001mol acetylferrocene, 0.001mol 2-amino-5-methyl-1,3,4-thiadiazole and 0.0012mol p-toluenesulfonic acid to a dry mortar, and grind at room temperature until the raw material The reaction was complete (monitored by TLC) for 25 minutes, and then kept in an oven at 50° C. for 30 minutes.
[0044] 2) Then cool it down to room temperature and add absolute ethanol, filter the mixture with suction to remove the catalyst p-toluenesulfonic acid, and concentrate the filtrate to dryness under reduced pressure to obtain the crude product. The crude product is recrystallized with absolute ethanol to obtain acetyl ferrocene 2-amino-5-methyl-1,3,4-thiadiazole.
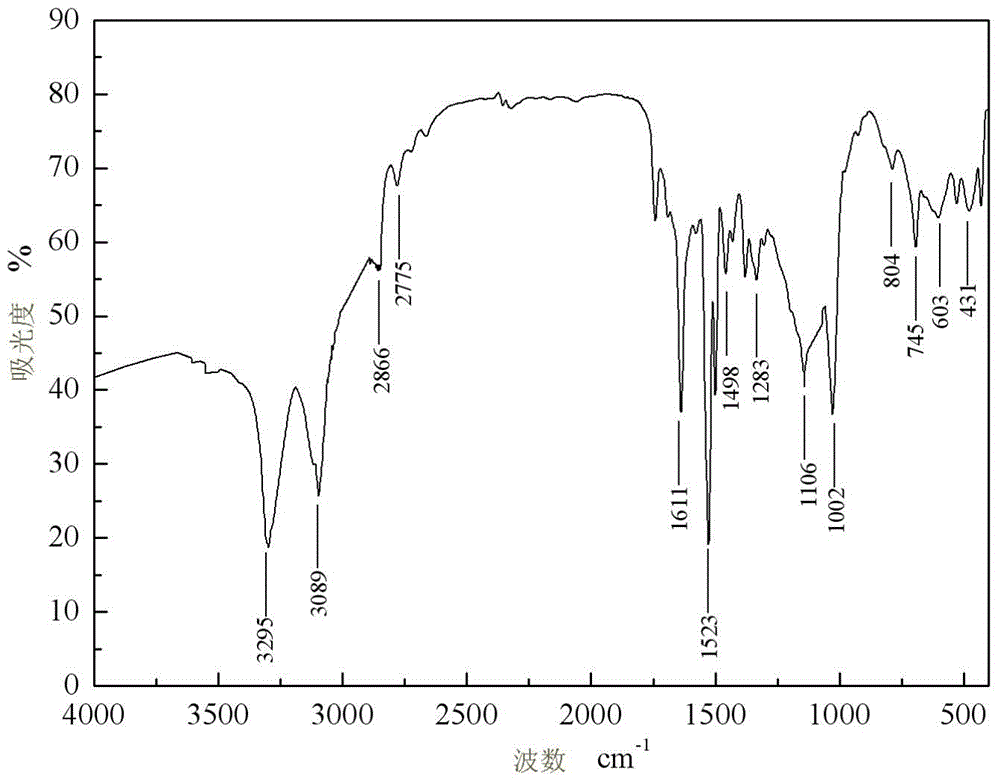
[0045] IR (KBr, v / cm -1 ): 3426, 2925, 1605, 1527, 1427, 1377, 1104, 1001, 828, 648, 495;
[0046] 1 H-NMR: 4.53-4.80 (2H, Fc-H, multiplet), 4.26 (5H, Fc-H, triplet), 2.05-2.52 (3H, -CH 3 ,Unimodal).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com