Novel crystal form of phosphorus-containing substituted quinazoline derivative as well as preparation method and application of novel crystal form
A technology of quinazoline and chlorination, which is applied in the field of new crystal forms of quinazoline derivatives and its preparation, can solve the problems of low bioavailability and blank basic drug research, and achieve improved bioavailability and improved drug production. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
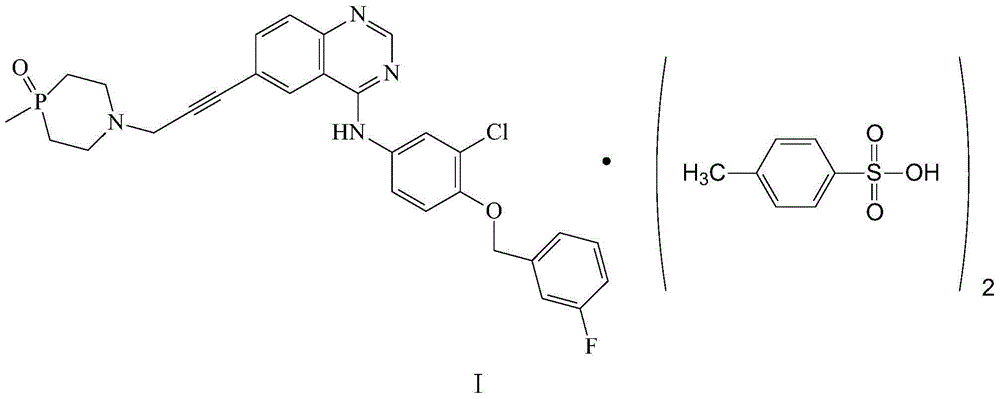
[0041] Embodiment 1: Preparation of N-(3-chloro-4-(3-fluorobenzoyloxy)phenyl)-6-(3-(4-methyl-1,4azaphosphine- 1-yl)prop-1-ynyl)quinazolin-4-amine p-toluenesulfonate crystal form
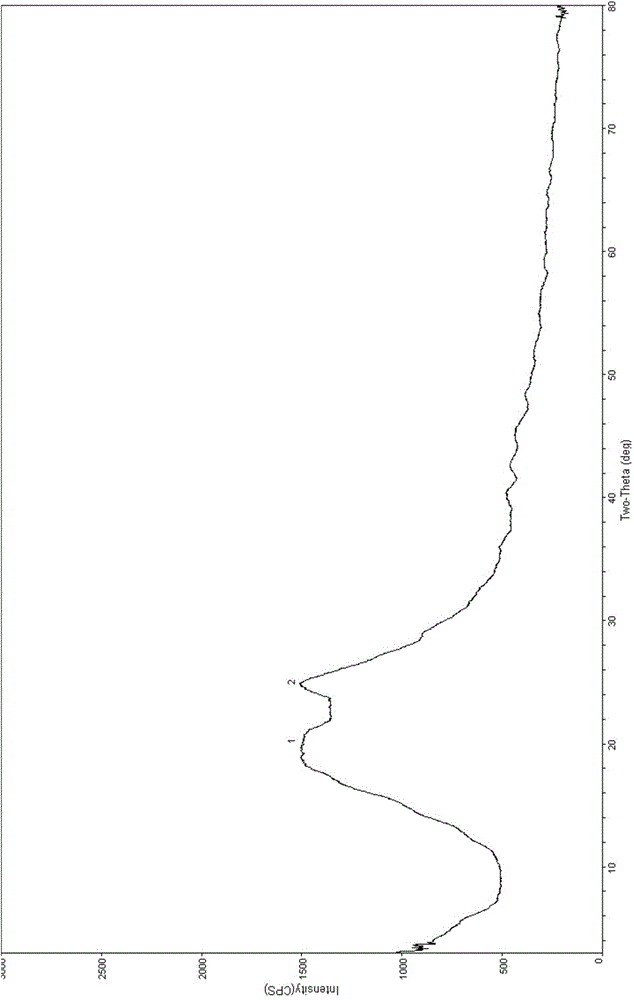
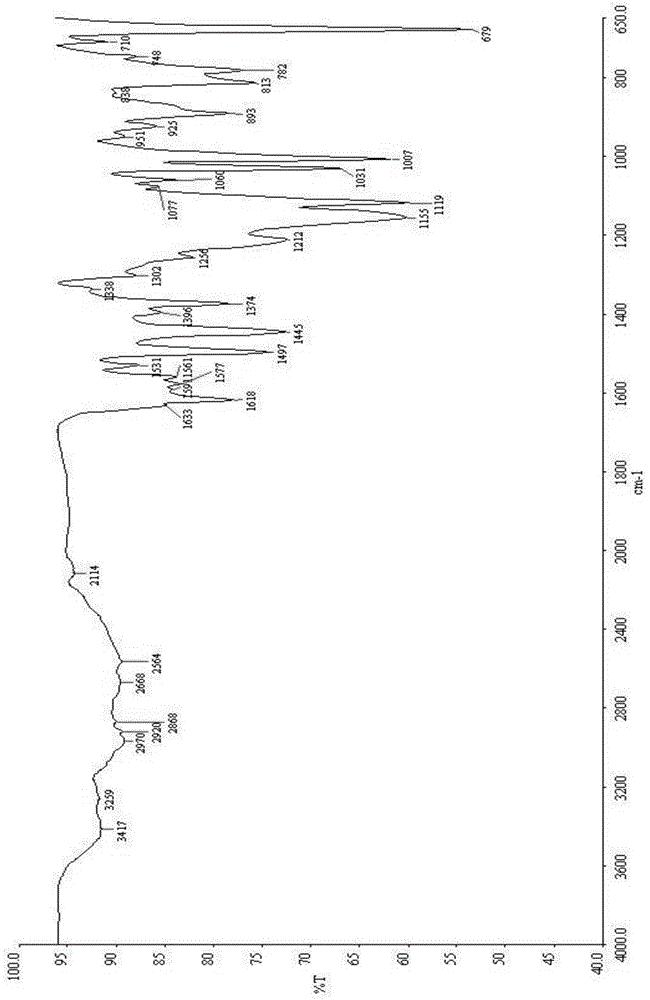
[0042] 100mg of N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-(3-(4-methyl-1,4azaphosphin-1-yl)propan-1- Alkynyl)quinazolin-4-amine p-toluenesulfonate sample was completely dissolved in 8.0mL mixed solvent (water:methanol volume ratio 1:2) at 40°C, and 88mg of the crystal form was obtained by cold spray method , carry out powder X-ray diffraction analysis to the obtained crystal form, and its diffraction pattern is as follows figure 1 As shown, the infrared spectrum as figure 2 shown.
Embodiment 2
[0043] Embodiment 2: Preparation of N-(3-chloro-4-(3-fluorobenzoyloxy)phenyl)-6-(3-(4-methyl-1,4azaphosphine- 1-yl)prop-1-ynyl)quinazolin-4-amine p-toluenesulfonate crystal form
[0044] 100mg of N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-(3-(4-methyl-1,4azaphosphin-1-yl)propan-1- Alkynyl)quinazolin-4-amine p-toluenesulfonate sample was completely dissolved in 10.0mL mixed solvent (water:ethanol volume ratio 1:1) at 60°C, and 84mg of the crystal form was obtained by cold spray method , powder X-ray diffraction analysis was performed on the obtained sample, and the diffraction pattern was basically as figure 1 As shown, the infrared spectrum is basically as figure 2 Shown, consistent with the identification result of embodiment 1.
Embodiment 3
[0045] Example 3: Preparation of N-(3-chloro-4-(3-fluorobenzoyloxy)phenyl)-6-(3-(4-methyl-1,4azaphosphine- 1-yl)prop-1-ynyl)quinazolin-4-amine p-toluenesulfonate crystal form
[0046] 100mg of N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-(3-(4-methyl-1,4azaphosphin-1-yl)propan-1- Alkynyl) quinazoline-4-amine p-toluenesulfonate sample was completely dissolved in 5.0mL mixed solvent (water:isopropanol volume ratio 1:3) at a temperature of 55°C, and the cold spray method was used to obtain 80mg of the crystal form, powder X-ray diffraction analysis was carried out on the obtained sample, and its diffraction pattern was basically as follows: figure 1 As shown, the infrared spectrum is basically as figure 2 Shown, consistent with the identification result of embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com