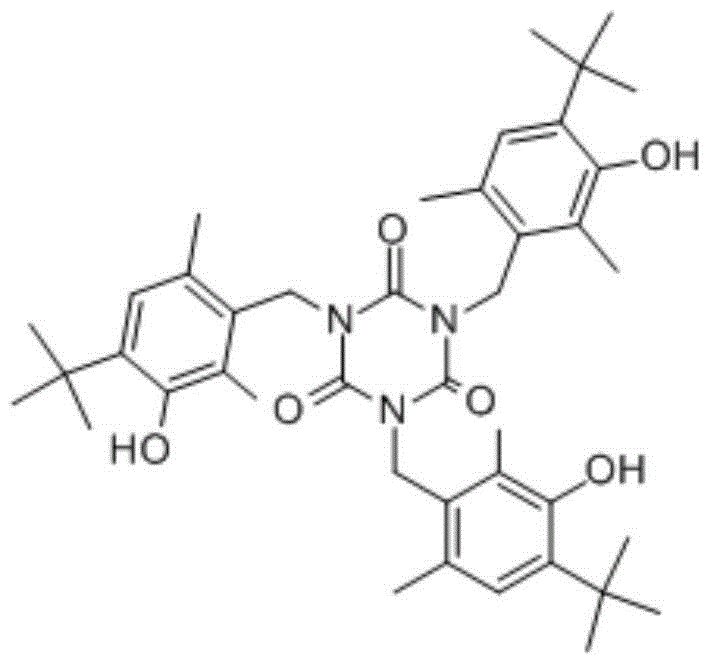
Synthetic method of 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione compound
A technology of dimethylbenzyl, synthesis method, applied in 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5- In the field of synthesis of triazine-2,4,6(1H,3H,5H)-triketone compounds, it can solve the problems of many by-products, high industrial energy consumption, and long reaction time, and achieve less by-products, low industrial energy consumption, The effect of short reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
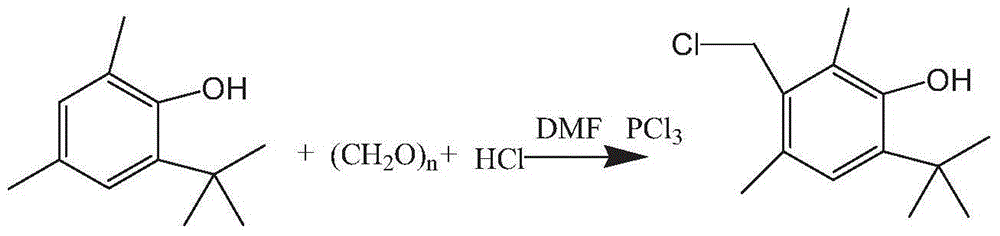
[0027] Embodiment 11, the synthesis of 4-tert-butyl-2,6-dimethyl-3-hydroxyl benzyl chloride
[0028] Add 49g of 32% hydrochloric acid and 49g of 2-tert-butyl-4,6-dimethylphenol at one time into the reaction flask, add 13.1g of paraformaldehyde under stirring, then heat up to 37°C, and keep it warm at this temperature for 2 After 1 hour, TLC followed the reaction to the end. After the heat preservation was finished, 17.3 g of phosphorus trichloride was started to be added dropwise, and the temperature of the kettle was controlled to 40° C., and the drop was completed in about 1 hour, and kept at this temperature for about 4 hours, and the TLC tracking reaction ended.
[0029] After the reaction, add 50g of toluene, stir for 15min and then let stand for 20min, separate the lower aqueous phase, wash the organic phase with saturated brine at 30°C (20ml x 2 times), evaporate the toluene under reduced pressure.
[0030] Cool to 35-40°C, and drop DMF under control at this temperatur...
Embodiment 21
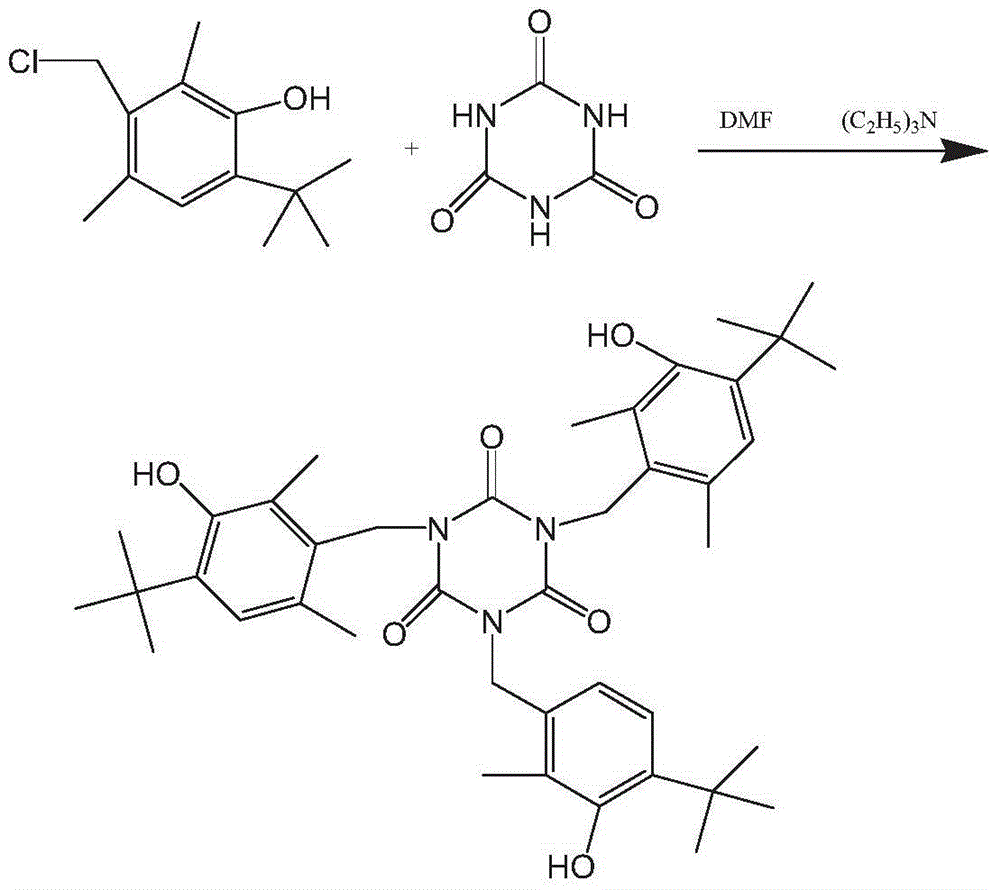
[0031] Example 21, 3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5-triazine-2,4,6(1H,3H, Synthesis of 5H)-triketone
[0032] Add 39g of DMF and 10.5g of cyanuric acid into the reaction bottle at one time, add 40.5g of triethylamine under stirring, and then heat up to 90°C, add the pre-prepared 1,4-tert-butyl-2,6- A mixture of 62.3 g of dimethyl-3-hydroxybenzyl chloride and 32.7 g of DMF was dropped into it. There is slight reflux during the process, and the temperature of the kettle will rise slowly, and it is required not to exceed 102°C, and the dripping will be completed in about 11 / 2 hours. Then incubate and react at 100-120° C. for about 5 hours, and follow up the reaction by TLC to complete.
[0033] After the reaction, the salt was removed by filtration at room temperature, rinsed with a small amount of DMF, and dried by filtration. Recover the solvent under reduced pressure, add 115g methanol, distill 60g under normal pressure reflux, drop to 1-2°C and kee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com